Niche-specific dermal macrophage loss promotes skin capillary ageing
Article metadata
Article Date: 15 October 2025
Article URL: https://www.nature.com/articles/s41586-025-09639-y
Article Image: Figure 1
Summary
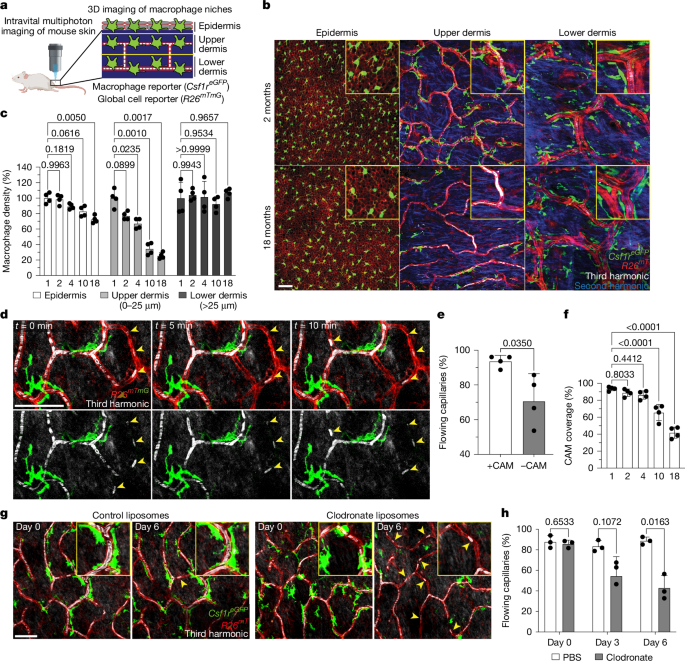
This Nature paper uses long-term intravital multiphoton imaging in mice, complementary genetic models and human tissue analysis to show that capillary-associated macrophages (CAMs) in the upper dermis decline with age. CAM loss occurs earlier and faster than capillary loss, and correlates with obstructed red blood cell flow and impaired capillary repair.
The authors demonstrate CAMs support microvascular function by rapidly recruiting to local clots, phagocytosing vascular debris (RAC1-dependent) and promoting reperfusion. CX3CR1 signalling guides CAM recruitment. Upper-dermal CAMs are maintained chiefly by local proliferation (not monocyte replacement), but homeostatic renewal is insufficient — so CAM density falls with age. Large local damage or intradermal CSF1–Fc restores CAM numbers and improves capillary perfusion in aged skin.
Methods include longitudinal live imaging of the mouse plantar skin, laser-induced capillary clotting and ablation, bone-marrow chimera and lineage tracing, Rac1 and Cx3cr1 genetic perturbations, clodronate and DTR depletion, and human histology comparisons.
Author style: Punchy. This is a mechanistic, high-quality in vivo study with clear translational implications for microvascular ageing and therapies that target tissue-resident macrophages.
Key Points
- Upper-dermal capillary-associated macrophages (CAMs) decline with age in mice and humans; their loss outpaces capillary disappearance.
- Capillary segments without CAMs show more obstructed red blood cell flow and are less likely to reperfuse after clotting.
- CAMs rapidly recruit to local capillary damage, engulf RBC/vascular debris and promote reperfusion; recruitment is partly CX3CR1-dependent.
- Phagocytosis by CAMs requires RAC1; RAC1-deficient CAMs show impaired debris clearance, slower reperfusion and accelerated capillary pruning.
- Upper-dermal CAMs are mostly host derived and sustained by local proliferation rather than monocyte replacement; homeostatic proliferation is insufficient to maintain density with age.
- CAM loss alone (without tissue damage) does not trigger efficient repopulation; large local damage or CSF1–Fc treatment expands CAM numbers.
- Local CSF1–Fc in aged mice increases CAM density and restores both baseline capillary flow and repair after induced clots — proof-of-principle for therapeutic niche rejuvenation.
Context and relevance
This study connects two major ageing themes: decline of tissue-resident immune cells and microvascular deterioration. Impaired capillary perfusion drives tissue ageing and disease (wound healing defects, sarcopenia, neurodegeneration), so identifying a local cellular mechanism — progressive erosion of CAM territory and function — is important. The work highlights that niche-specific renewal strategies (local proliferation versus monocyte replacement) shape vulnerability to ageing and that boosting the niche (CSF1) can be beneficial.
Why should I read this?
Short version: if you care about how microvessels fail with age, wound healing or immune–tissue interactions, this paper is worth your time. It’s a neat combination of live imaging, genetics and human validation that shows resident macrophages actively guard capillary function — and that you can re-expand them locally to reverse age-linked microvascular dysfunction. We’ve saved you the deep read: key mechanisms are CX3CR1-guided recruitment, RAC1-dependent phagocytosis and CSF1-driven niche expansion.