SARS-CoV-2 mRNA vaccines sensitize tumours to immune checkpoint blockade
Article Date: 22 October 2025
Article URL: https://www.nature.com/articles/s41586-025-09655-y
Article Image: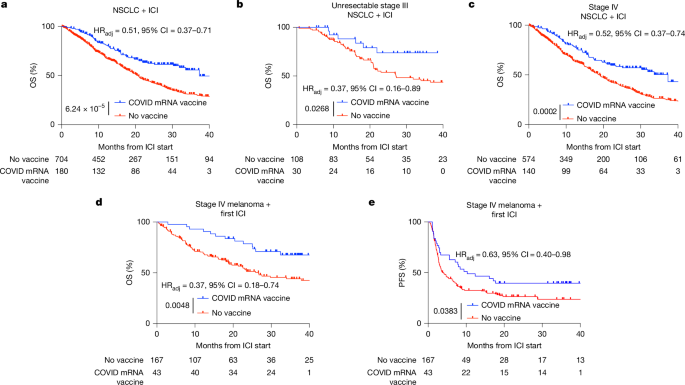
Summary
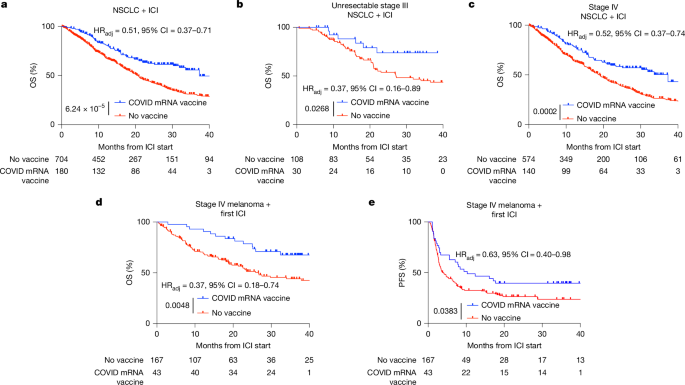
This Nature paper shows that commercially available SARS-CoV-2 mRNA vaccines (the COVID-19 mRNA platform) can reset systemic and intratumoural immunity and sensitize otherwise resistant tumours to immune checkpoint inhibitors (ICIs). Retrospective clinical analyses at MD Anderson found markedly better overall survival (OS) for patients with non-small cell lung cancer (NSCLC) and metastatic melanoma who received a COVID-19 mRNA vaccine within 100 days of starting ICI. In parallel, mouse models reproduced the effect: spike mRNA lipid nanoparticles (RNA-LNPs) produced a surge in type I interferon (IFN-I), activated antigen-presenting cells (APCs) in lymphoid organs, primed tumour-reactive CD8+ T cells, increased tumour PD-L1 and—when combined with ICIs—led to tumour regression and epitope spreading. Human volunteer sampling confirmed transient IFNα surges and innate/adaptive activation after vaccination. Pathology data showed increased tumour PD-L1 expression (TPS) within 100 days of mRNA vaccination, suggesting the vaccines broadly amplify the ICI-relevant immune cycle.
Key Points
- Receipt of a SARS-CoV-2 mRNA vaccine within 100 days of starting ICI was associated with significantly improved OS in NSCLC and metastatic melanoma cohorts (adjusted HRs ~0.37–0.52; strong statistical controls applied).
- Preclinical spike RNA-LNPs synergise with anti-PD-1/PD-L1 in mice, reducing tumour growth and metastases in otherwise ICI‑resistant models (B16F0, LLC).
- Type I interferon (IFNα) is necessary for the antitumour effect—blocking IFNAR1 abolishes benefit; exogenous high-dose IFNα can partially phenocopy the effect.
- RNA-LNPs rapidly activate APCs in spleen and tumour‑draining lymph nodes, boosting antigen presentation (especially Ly6C+ MHC‑II+ myeloid cells) and priming tumour‑reactive CD8+ T cells with evidence of epitope spreading.
- Vaccination triggers increased PD-L1 expression on tumour and myeloid cells — a compensatory change that makes tumours more targetable with ICIs but requires concurrent checkpoint blockade to sustain effector T cells.
- Human data (healthy volunteers) show a short-lived but large IFNα surge (peak ~24 h), innate-cell activation and transient increases in PD-L1 on circulating myeloid/DC populations after mRNA vaccination.
- Pathology review across diverse histologies found higher tumour PD-L1 TPS within 100 days of mRNA vaccination (NSCLC and tissue‑agnostic cohorts); influenza/pneumococcal vaccines did not show this effect.
Content summary
The authors combined rigorous retrospective clinical analyses (NSCLC, melanoma, and a tissue-agnostic PD-L1 cohort) with mechanistic preclinical work and controlled human sampling. Clinically, vaccination near ICI initiation correlated with doubled 3‑year OS in some NSCLC analyses and large improvements in melanoma OS/PFS after adjustment and propensity matching. Mechanistically, commercially formulated spike mRNA in LNPs induced high‑molecular‑weight secondary RNA structures, stimulated MDA5/IFN-I pathways, and produced a systemic cytokine/chemokine surge that activated APCs and primed CD8+ T cells. Tumour infiltration by PD‑1+CD8+ cells and increased PD‑L1 on tumour cells were prominent; combining vaccination with ICIs overcame PD‑L1‑mediated compensation and drove tumour rejection in models. Human volunteers showed parallel short-term IFNα and innate/adaptive activation. Overall, the data support off‑the‑shelf mRNA vaccines as immune modulators that can sensitize immunologically “cold” tumours to ICIs.
Context and relevance
This is important for clinicians and researchers working on cancer immunotherapy because it reveals a widely available, low‑cost, immediately deployable method to boost ICI responses: routine mRNA vaccination around the time of ICI start. It helps explain anecdotal tumour regressions after COVID vaccination, provides mechanistic evidence tying IFN‑I‑driven APC priming to epitope spreading, and suggests that timing of vaccination could affect treatment decisions (for example, PD‑L1 TPS thresholds used to select single‑agent immunotherapy). It also opens translational avenues for purpose‑designed off‑the‑shelf RNA therapeutics to reset immune setpoints before ICI.
Why should I read this?
Short version: if you care about making immunotherapy work for more patients, this paper matters. It shows a real, clinically observed survival boost tied to a mechanism you can understand and, crucially, potentially act on now. We’ve saved you the deep read — key takeaway: time an mRNA vaccine around ICI start and the immune system might just do the heavy lifting it usually refuses to do.
Author note (style)
Author style: Punchy — the study is clinically actionable and mechanistically convincing; the authors amplify that this could be a practical, off‑the‑shelf strategy to overcome intrinsic ICI resistance.