Evidence for improved DNA repair in long-lived bowhead whale
Article meta
Article Date: 29 October 2025
Article URL: https://www.nature.com/articles/s41586-025-09694-5
Article Image: Figure 1
Summary
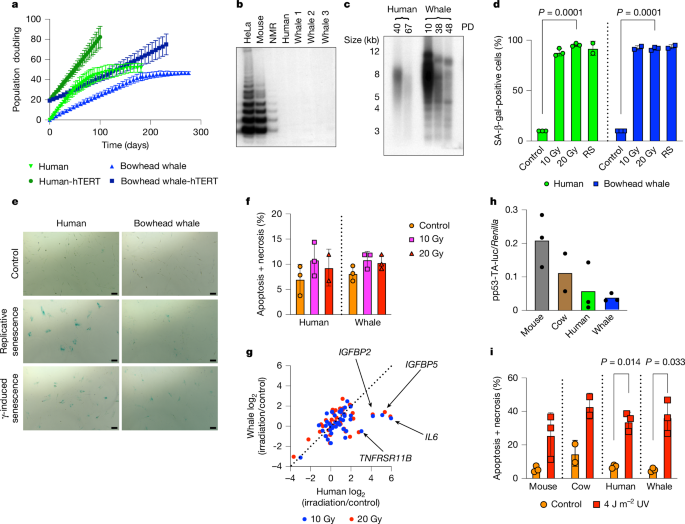
This Nature paper investigates cellular and molecular reasons behind the extraordinary longevity (>200 years) and apparent low cancer incidence of the bowhead whale (Balaena mysticetus). Using primary fibroblasts, tumourigenicity assays, whole-genome sequencing, DNA repair measurements, proteomics and in vivo fly experiments, the authors show that bowhead whale cells accumulate fewer mutations, especially large structural variants, and have enhanced, more accurate DNA repair — notably double-strand break (DSB) repair and mismatch repair. A cold-inducible RNA-binding protein, CIRBP, is highly abundant in bowhead tissues and contributes to efficient and accurate DSB repair. Expression of bowhead CIRBP (bwCIRBP) improves repair fidelity in human cells and increases resistance to DNA damage and lifespan in Drosophila models. The study argues that improved maintenance of genome integrity, rather than hyperactive apoptosis or expanded tumour suppressor copy number, is a key strategy the bowhead whale uses to resolve Peto’s paradox and achieve exceptional lifespan.
Key Points
- Bowhead whales live >200 years yet do not show expected high cancer rates for such large, long-lived mammals (Peto’s paradox).
- Bowhead skin fibroblasts undergo normal replicative senescence, lack telomerase activity in most tissues and show an attenuated SASP (less inflammatory senescent phenotype) relative to human cells.
- Bowhead fibroblasts require fewer engineered tumour-suppressor hits for transformation in fibroblast models than human fibroblasts, but paradoxically display much lower spontaneous and induced mutation rates.
- Whole-genome sequencing of tumours and reporters shows bowhead cells accumulate significantly fewer SNVs, indels and especially large structural variants (SVs) than human or mouse cells after transformation or mutagen exposure.
- Multiple DNA repair pathways are improved in bowhead cells: mismatch repair is stronger, PARP activity is higher, and both non-homologous end joining (NHEJ) and homologous recombination (HR) DSB-repair frequencies are elevated with faster resolution of DSB foci.
- NHEJ in bowhead cells is not just more active but more accurate: fewer deletions and fewer large deletion outcomes at CRISPR-induced breaks were observed compared with other mammals.
- CIRBP is highly abundant in bowhead fibroblasts and tissues; high CIRBP levels promote NHEJ and HR, protect DNA ends from resection, reduce indels and chromosomal aberrations, and knockdown reduces repair efficiency.
- Overexpressing bwCIRBP in human cells improves genome stability and delays anchorage-independent growth; expressing CIRBP in Drosophila extends lifespan and increases radiation resistance.
- The authors propose that enhanced, high-fidelity DNA repair and genome maintenance — assisted by abundant CIRBP — is a conservative strategy that repairs rather than eliminates cells, helping explain longevity and cancer resistance in bowhead whales.
Context and relevance
This work addresses Peto’s paradox by showing that large, long-lived animals can evolve genome-maintenance strategies distinct from the tumour-suppressor duplications seen in elephants. Rather than relying primarily on increased apoptosis or expanded tumour-suppressor copy number, bowhead whales appear to invest in repair fidelity and reduced mutation accumulation. That has twofold relevance: (1) it advances comparative biology of ageing and cancer resistance, and (2) it identifies molecular players (notably CIRBP) that could become targets for research into therapies to improve genome stability in humans or to treat genome-instability conditions.
Author take (punchy)
Punchline: the longest-lived mammal we studied keeps its genome clean rather than just killing damaged cells. The big surprise is CIRBP — a cold‑inducible RNA-binding protein — turned out to be a repair booster. This isn’t just evolutionary trivia: the paper gives clear experimental leads (bwCIRBP improves repair in human cells; it helps flies live longer and resist radiation) that make the bowhead’s approach to longevity actionable for further translational research.
Why should I read this?
Because if you care about why some animals age so slowly or how nature beats cancer risk at scale, this is a neat, experimentally solid clue. The team read the fine print in whale cells for you: they show fewer mutations, better double‑strand break repair and a standout protein (CIRBP) that actually improves repair when moved into other species. If you’re into ageing biology, cancer biology, or DNA repair therapeutics, it’s worth the 10–15 minutes to dig into their figures and methods.
Limitations & notes
The cell-based transformation assays were performed in fibroblasts (not epithelial cells, where most human cancers originate), and mechanistic details of exactly how CIRBP promotes accurate end-joining (LLPS, RNA- or PAR-dependent scaffolding) need more work. Yet the data present a consistent cross-platform story (genomics, proteomics, functional assays, and in vivo fly work).
Source
Original article: https://www.nature.com/articles/s41586-025-09694-5