Multi-omic profiling reveals age-related immune dynamics in healthy adults
Summary
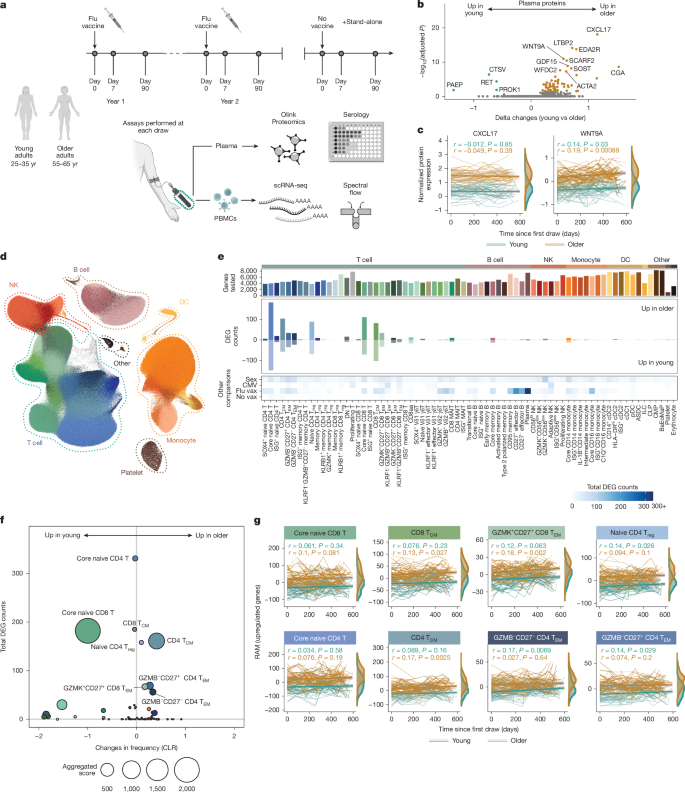
This large multi-omic, longitudinal study (the Sound Life Project and a follow-up cohort) tracks peripheral immune changes across adulthood using single-cell RNA sequencing (>13.7 million PBMCs), high-dimensional proteomics and spectral flow cytometry. The teams compared younger adults (25–35) with those at the cusp of ageing (55–65) over two years and extended analyses across a 40–90+ follow-up cohort.
Key discoveries: transcriptional reprogramming occurs early and stably in T cells (especially naive and central memory subsets) before advanced age; a novel RNA Age Metric (RAM) captures subset-specific transcriptional ageing; memory T cells progressively skew towards a T H 2-like state driven by increased GATA3 activity and IL-4 production; these T cell changes associate with altered B cell class-switching (IgG2/IgG3 skewing) and reduced functional antibody responses to repeatedly encountered influenza antigens (notably B/Phuket). By contrast, chronic CMV reshapes cell composition (adaptive NK and terminal effector T cells) but does not drive the same transcriptional ageing programme.
Key Points
- Longitudinal multi-omic profiling across 96 deeply sampled adults and a 234-person follow-up produced a high-resolution immune atlas covering millions of single cells.
- T cells show the largest and most stable age-related transcriptional changes prior to advanced ageing; naive CD4 and CD8 and central memory subsets are most affected.
- RNA Age Metric (RAM) summarises subset-specific transcriptional ageing and correlates with existing immune-age metrics (IMM-AGE and IHM).
- Memory T cells progressively acquire a T H 2-like programme with age (increased GATA3 motif activity and IL-4 production), evident in blood and lymph node samples.
- T H 2 skewing correlates with altered memory B cell activation, reduced IgG class-switching in a key CD27− effector memory B subset, and lower functional haemagglutination-inhibition (HAI) responses to repeatedly boosted influenza strain B/Phuket.
- CMV infection produces stable expansions of terminal effector T cells and adaptive NK cells but does not accelerate T cell transcriptional ageing (RAM unchanged by CMV).
- Age-related immune changes occur without broad systemic inflammation (no consistent increase in TNF, IL-6 or IL-1B prior to advanced age).
- RAM can flag accelerated immunological ageing in disease-risk contexts (example: raised RAM in CD4 central memory cells in preclinical rheumatoid arthritis).
Context and relevance
This paper matters because it maps when and how immune programmes shift before overt decline in older age. Instead of a uniform inflammatory ‘decline’, the study shows a targeted and stable transcriptional reprogramming in early-differentiated T cells and a functional link to weakened antibody responses following repeated antigen exposure. That has immediate relevance for vaccine strategy, immunotherapy timing and understanding mechanisms behind age-associated autoimmunity and infection susceptibility.
Author style (punchy)
This is a heavyweight data resource with clear mechanistic signals — T cells change first, not last. If you care about why vaccines underperform in later life or how immune tolerance shifts with age, the detailed figures and the new RAM score are worth a deep dive.
Why should I read this?
Short version: if you’re strapped for time — read this because it shows that immune ageing starts earlier than you might think, is driven mainly by T cell reprogramming (not general inflammation), and directly alters B cell responses and antibody quality to repeated vaccines. Handy if you want evidence to tweak vaccine design, timing or immune-monitoring approaches.
Source
Source: https://www.nature.com/articles/s41586-025-09686-5
Notes
Data and code are openly available (GEO, dbGaP and GitHub links in the paper) and the authors provide interactive explorers (Human Immune Health Atlas, Sound Life visualisers) for deeper inspection.