Ultrasound-driven programmable artificial muscles
Summary
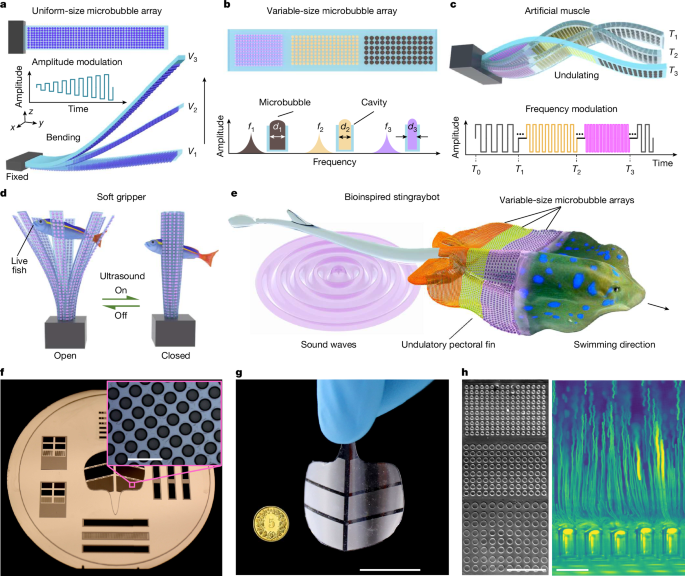
Researchers present soft artificial muscles that use densely patterned microbubble arrays trapped in thin PDMS membranes and driven by ultrasound. Resonant microbubbles concentrate acoustic energy to produce microstreaming and a reverse thrust that bends the membrane. By patterning cavities with different diameters (hence different resonance frequencies) and using sweeping-frequency ultrasound, the team achieves frequency-selective, programmable, multimodal deformations — from rapid uniform flexing to undulatory motions. Fabricated by soft lithography, prototypes include a millisecond-response soft gripper, conformable robotic skin and a bioinspired “stingraybot” that swims and can be deployed from edible capsules into ex vivo porcine organs. The authors provide a theoretical model linking microbubble streaming to thrust and beam deflection, report force intensities (≈7.6 μN mm−2) and sub-100 ms response times, and test performance in blood and other fluids. Limitations include bubble growth under prolonged actuation (~30 min) and distance-dependent attenuation of ultrasound, with proposed fixes such as cavity sealing and optimised ultrasound delivery.
Key Points
- Microbubble arrays (10,000s per actuator) trapped in PDMS cavities convert ultrasound into localised reverse thrust via acoustic streaming.
- Different cavity sizes resonate at distinct frequencies, enabling frequency-selective activation and programmable multimodal deformation.
- Sweeping-frequency ultrasound produces coordinated undulatory motions used to propel a soft “stingraybot” and drive other robots.
- Prototypes demonstrated: a fast soft gripper (grasps zebrafish larvae in ~100 ms), conformable robotic skin, and swallowable/swelling deployable actuators in ex vivo porcine organs.
- Theoretical model: thrust from each bubble scales with cavity radius and oscillation velocity (Fi ≈ πρ ω Rc,i3 vi), and beam deformation follows linear-elastic beam theory; deformation scales approximately with VPP2 and with bubble density.
- Actuators work in physiologically relevant fluids (including 100% porcine blood) and across scales from microscale to centimetre devices.
- Main limitations: microbubble growth destabilises long runs (~30 min), and ultrasound intensity decays with distance — mitigations include sealing cavities and optimising transducer geometry.
- Potential applications span soft robotics, wearable haptics, minimally invasive surgical devices and targeted in vivo drug delivery.
Why should I read this?
Want wireless, tiny, fast artificial muscles that actually work in biological settings? This paper shows a clever, practical route: pack a flexible membrane with tuned microbubbles, hit it with ultrasound and you get precise, programmable motion without tethers or bulky magnets. If you care about soft robots inside the body, swallowable devices or ultrasound‑compatible actuators, this is the one to skim — we read it so you don’t have to (but it’s worth digging into the figures).
Author’s take
Punchy and important. This isn’t a marginal materials tweak — it’s a systems-level demonstration of wireless, frequency‑selective soft actuation with clear in‑ex vivo proof‑of‑concepts. For anyone building biomedical soft robots or compact wearable actuators, the approach is immediately relevant and ripe for follow‑up.
Context and Relevance
Ultrasound offers deep tissue penetration, clinical compatibility and millisecond control without invasive hardware. By harnessing resonant microbubbles embedded in soft polymers, the work bridges acoustofluidics and soft robotics to overcome the usual drawbacks of tethered pneumatic or magnetic systems. The ability to program spatially local forces with frequency sweeps opens design space for in vivo mechanotherapy, site‑specific drug delivery and minimally invasive robotic tools that can be delivered via capsules or endoscopes. The study addresses current trends towards untethered, biocompatible actuation and provides a design and modelling framework to scale and optimise performance.
Source
Source: https://www.nature.com/articles/s41586-025-09650-3
Article metadata
Article Date: 29 October 2025
Article Image: Figure 1