Synthetic α-synuclein fibrils replicate in mice causing MSA-like pathology
Article Date: 05 November 2025
Article URL: https://www.nature.com/articles/s41586-025-09698-1
Article Image: Figure 1 (source)
Summary
This Nature paper reports a synthetic α-synuclein (aSyn) fibril strain, called 1B, whose atomic structure was solved (1.94 Å) and whose in vivo seeding product (1B P) was solved at 3.8 Å. The authors show that 1B fibrils seed aSyn pathology in wild-type (WT) mice that is highly reminiscent of multiple system atrophy (MSA) — producing neuronal and oligodendroglial inclusions (NCIs, NIIs, GCIs, GNIs) and spreading through myelinated tracts. In M83 (human A53T SNCA) transgenic mice 1B causes rapid, fatal disease and abundant MSA-like inclusions. Cryo-EM, CLEM, immunogold labelling and h-FTAA FLIM indicate that the seeded fibrils in vivo (1B P) closely replicate the synthetic seed’s fold. The 1B/1B P fold shares a kernel with previously reported patient-derived MSA filaments, notably featuring closed N- and C-pockets (which make these fibrils ThT-invisible). Brain homogenates containing 1B P can re-seed MSA-like pathology in WT mice, supporting a protein-only templating (prion-like) replication mechanism.
Key Points
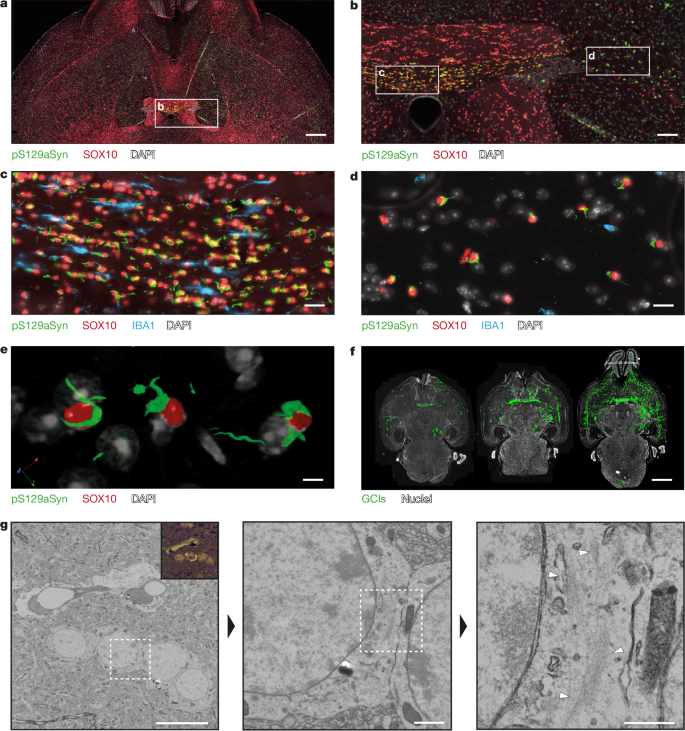
- 1B is a synthetic α-synuclein fibril strain whose atomic structure was solved to 1.94 Å; the in vivo product (1B P) was solved at 3.8 Å, showing high structural similarity.
- Intracerebral inoculation of 1B in WT mice produces MSA-typical pathology: neuronal inclusions early, then abundant glial cytoplasmic inclusions (GCIs) in myelinated tracts over months.
- In M83 (A53T human aSyn) mice, 1B induces rapid, synchronised fatal disease within ~16 weeks with extensive GCIs and glial nuclear inclusions.
- CLEM, electron tomography and immunogold confirm fibrillar aSyn material within inclusions; h-FTAA FLIM shows conformational similarity between 1B-seeded mouse inclusions and human MSA inclusions.
- The 1B/1B P fold contains a pseudo-Greek-key motif and closed N- and C-pockets — structural features that explain ThT-negativity and correlate with seeding potency.
- Crude brain homogenates containing 1B P (no detergent purification) can re-seed MSA-like pathology in WT mice, demonstrating transmissible templated replication in vivo.
- Mutational analysis shows seeding is sensitive to disruption of the pseudo-Greek-key stabiliser (E46K), suggesting specific fold elements encode strain behaviour and could be targeted.
Context and relevance
MSA is a rapidly progressive synucleinopathy defined by oligodendroglial inclusions (GCIs). Whether aSyn assemblies can act as self-replicating, strain-like agents driving MSA pathology has been debated. This study provides high-resolution structural and in vivo evidence that a fully synthetic aSyn fibril strain can faithfully template its fold in mice and induce MSA-like neuropathology. That links atomic-level fibril architecture (the “MSA kernel” and closed pockets) to biological behaviour — seeding specificity, ThT invisibility and high pathogenicity. For researchers this is important because it clarifies which structural features correlate with aggressive, GCI-producing strains and suggests mechanistic targets (pocket opening, Ser87 modification) for interventions or diagnostics. It also reinforces biosafety caution when working with certain synthetic fibrils because of their transmissible properties.
Why should I read this?
Quick and blunt — if you work on synuclein, prion-like spread, neurodegenerative mechanisms or structural biology, this paper is a must-read. It shows a synthetic fibril can replicate its exact fold in vivo, cause MSA-typical inclusions and be transmissible between animals. It connects high-resolution structure to disease-relevant behaviour and flags concrete structural hotspots that might be druggable. If you don’t work in the field, you’ll still want to know: it changes how we think about strain-driven α-synuclein disease and lab safety.
Author style
Punchy — the authors present decisive structural and in vivo data that amplify the importance of strain-specific aSyn conformations for MSA pathology. The work is a strong demonstration of protein-only templating in a mammalian brain and has immediate implications for experimental practice and therapeutic strategy.