Florigen activation complex forms via multifaceted assembly in Arabidopsis
Article meta
Article Date: 12 November 2025
Article URL: https://www.nature.com/articles/s41586-025-09704-6
Article Image: Figure 1
Summary
This Nature paper dissects how the florigen activation complex (FAC) assembles in Arabidopsis to trigger flowering. Using fluorescent genomic fusions, RNAscope, in vivo IP–MS and extensive in vitro biochemistry, the authors map where and when FLOWERING LOCUS T (FT), the bZIP transcription factor FD and multiple 14-3-3 isoforms come together at the shoot apical meristem (SAM) and in emerging floral primordia. Key mechanistic findings are: phosphorylation of FD at T282 enables binding to 14-3-3 proteins; 14-3-3s act as chaperone-like partners that prevent FD forming large condensates, promote FD dimerisation and enhance its DNA binding; FT recruitment to the complex requires DNA-bound FD–14-3-3, with the FT C-terminal tail making direct contacts with DNA (interface 1) plus a conserved interface (interface 2) engaging 14-3-3. Mutations that disrupt these interfaces impair FAC assembly and delay flowering. The authors present a model in which FAC formation is a stepwise, DNA-guided and phosphorylation-regulated process that integrates protein–protein interactions and phase-separation control to activate floral genes such as SEP3 and AP1.
Key Points
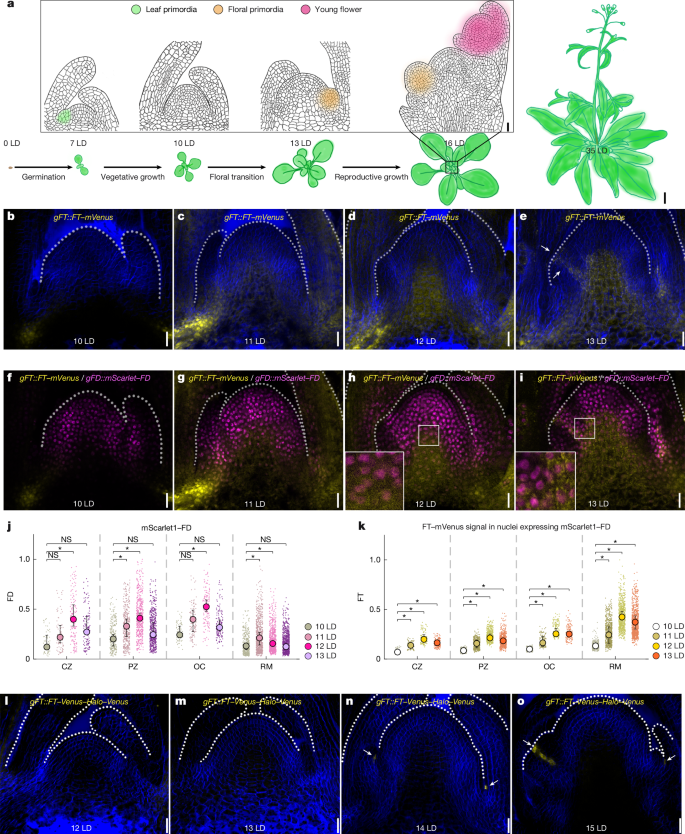
- FT protein accumulates at the SAM and in floral primordia; initially FT moves from leaf companion cells, later local FT transcription appears in primordia.
- FD and multiple 14-3-3 isoforms co-localise with FT at the SAM, providing the spatial context for FAC assembly during floral induction.
- FD is phosphorylated at T282 in vivo; this phosphorylation is essential for 14-3-3 binding and for FD function in promoting flowering.
- 14-3-3 proteins suppress FD liquid-like condensation, promote FD dimerisation and increase FD DNA-binding — acting in a chaperone-like, multi-level regulatory role.
- FT does not strongly bind 14-3-3 alone in Arabidopsis; FT recruitment requires the FD–14-3-3 complex bound to DNA, revealing DNA as a structural determinant of FAC assembly.
- The unstructured FT C-terminal tail (positively charged residues R166, R173, R174) contacts DNA (interface 1); mutation of these residues (Mu1) abolishes FT recruitment and floral promotion.
- A second conserved interface (interface 2) mediates FT–14-3-3 contacts; mutations here (Mu2) reduce but do not abolish function, indicating both interfaces contribute to biological activity.
- The assembled FAC stabilises binding to target promoters (for example SEP3) and activates transcription of floral identity genes; disrupting assembly delays or prevents flowering.
- The study links classical florigen biology with modern concepts of biomolecular condensation and shows how modulation of condensation is functionally important for transcription factor activity in plants.
Content summary
Using a functional genomic FT::FT-mVenus fusion in ft-10 mutants and RNAscope for mRNA localisation, the authors mapped FT protein and transcript dynamics across long-day floral induction. FT appears first in vasculature and then in provasculature and specific SAM regions; later, FT mRNA is transcribed locally in primordia. Parallel imaging of FD and 14-3-3 fusions showed nuclear co-localisation that permits FAC formation.
Biochemically, IP–MS found many 14-3-3s associated with FD but not FT, suggesting FT interacts transiently. Recombinant protein work revealed FT alone does not stably associate with 14-3-3 or FD in solution; however, when FD–14-3-3 is bound to FD-target DNA, FT is recruited. Structural modelling and mutational analysis define two interfaces: (1) the FT tail contacting DNA directly and (2) a conserved FT surface engaging 14-3-3. FT Mu1 (tail arginines mutated) fails to be recruited and cannot rescue ft mutants; Mu2 partially retains function.
Crucially, FD has intrinsically disordered regions and forms phase-separated droplets in vitro when unregulated. Phosphorylation at T282 allows 14-3-3 binding, which prevents excessive FD condensation, promotes correct FD dimerisation and enhances specific DNA binding. Mutations that block phosphorylation or 14-3-3 interaction cause FD condensates, reduced DNA binding and loss of transcriptional activation of FD targets.
Context and relevance
This work resolves long-standing questions about how FT (the florigen) is recruited to transcriptional complexes at the SAM and clarifies the roles of 14-3-3 proteins beyond simple adapters. It connects florigen biology with phase-separation mechanisms increasingly recognised in transcriptional control. For researchers working on flowering time, crop breeding or transcription factor regulation, the paper identifies tangible molecular interfaces (FT tail and conserved FT/14-3-3 contacts; FD phosphorylation site) that could be targeted to alter flowering or meristem fate. The findings are also likely broadly relevant across seed plants given conservation of PEBP, bZIP and 14-3-3 families.
Why should I read this?
Because if you care about how plants decide when to flower, this paper actually explains the nuts-and-bolts: where FT shows up, how FD is readied (phosphorylation + 14-3-3), why FT only joins up on DNA, and why stopping FD clumping matters. It’s like they opened the black box of florigen assembly — handy if you want to tinker with flowering time or understand phase separation in plant transcription factors.
Author style (punchy)
Punchline: FAC formation is not a one-on-one handshake — it’s a choreographed, DNA-guided, phosphorylation‑dependent assembly in which 14-3-3s act as chaperones. If you work on flowering, meristem identity or TF phase behaviour, the detailed mechanistic map here is directly actionable — this is worth reading slowly and saving the figures.