Spatial fibroblast niches define Crohn’s fistulae
Article Date: 12 November 2025
Article URL: https://www.nature.com/articles/s41586-025-09744-y
Article Image: Figure 1
Summary
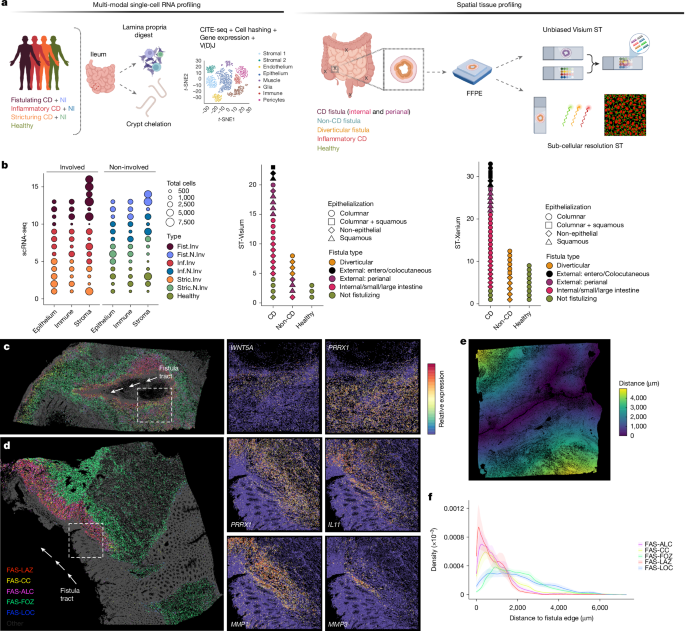
This Nature paper maps the cellular and molecular architecture of Crohn’s disease (CD) fistulae using high-resolution spatial transcriptomics, single-cell RNA-seq, multiplexed immunofluorescence and quantitative collagen imaging across 92 individuals. The team identifies a distinct group of fibroblasts—termed fistula-associated stromal (FAS) cells—that form layered niches around fistula tracts. Different FAS subtypes occupy discrete spatial zones with specialised programmes: superficial repair/remodelling, proliferative lesion cores and deeper fibrotic outer zones. Developmental transcription factors (TWIST1, OSR2, PRRX1, etc.) and morphogen/PCP signalling (WNTs, Frizzleds, PCP components) are implicated in driving invasive, remodelling and fibrotic behaviours. FAS niches interact closely with SPP1+ macrophages and neutrophils, and distinct ECM signatures correlate with tract persistence versus re-epithelialisation. Functional perturbation in primary fibroblasts (TWIST1/OSR2 overexpression) recapitulated key programmes linked to fibrosis or epithelialisation, suggesting targetable pathways.
Key Points
- Researchers profiled 129,204 single cells and >7 million segmented cells from spatial assays to create a spatially resolved atlas of intestinal fistulae.
- They discovered a novel population—FAS (fistula-associated stromal) fibroblasts—enriched in fistulating CD and organised in layered niches (LAZ, ALC, FOZ, LOC).
- FAS subtypes show zonated gene programmes: superficial remodelling and immune recruitment, proliferative/morphogen signalling at lesion edges, and deeper pro-fibrotic collagen deposition stabilising tracts.
- TWIST1 and OSR2 are key transcriptional regulators: TWIST1 drives pro-fibrotic ECM and MMP programmes; OSR2 activates patterning/PCP and matrix-remodelling programmes linked to epithelialisation.
- SPP1+ macrophages and chemokine networks (CXCLs, CCLs) create inflammatory–stromal niches that sustain recruitment and ECM turnover at the lumen surface.
- Picrosirius red imaging shows discrete hypo-dense collagen at lumen edges (remodelling) versus dense, anisotropic fibrotic bundles deeper in tracts (stabilisation).
- FAS-like fibroblasts appear at ulcer bases broadly, but in fistulae they become more abundant and reprogrammed toward invasive, pro-fibrotic states—suggesting ulcer-to-fistula transitions involve reprogramming rather than entirely distinct origins.
- Data and processed resources are publicly available (GEO and Mendeley Data) and the findings highlight potential stromal targets to limit tract formation or promote resolution.
Content summary
The authors assembled a large, multi-centre cohort of fistula and control tissues and applied Visium and Xenium spatial transcriptomics alongside pan-lineage scRNA-seq. Integrative analysis annotated broad cell populations and identified nine fibroblast subsets, including FAS cells unique to fistulae. Spatial maps revealed that FAS cells localise precisely at fistula edges and form layered niches with distinct molecular functions—remodelling and immune recruitment at the surface, proliferative/morphogen signalling at lesion fronts, and collagen-rich fibrotic zones deeper in the stroma.
Meta-analysis across many IBD single-cell datasets confirmed FAS signatures are enriched in fistulating CD and overlap partially with inflammation-associated fibroblasts, but with unique wound-healing, morphogen and fibrotic programmes. Overexpression experiments in primary intestinal fibroblasts showed TWIST1 induces ECM/fibrosis programmes while OSR2 drives PCP and remodelling programmes and can induce TWIST1, suggesting hierarchical transcriptional control. Spatial receptor–ligand and niche analyses highlight macrophage–fibroblast cross-talk, chemokine-driven recruitment, angiogenesis and WNT/PCP signalling at invasive leading edges. Collagen imaging aligned FAS subtypes with local ECM structure: remodelling zones beneath the lumen are hypo-dense while FOZ regions correspond to rigid, parallel collagen bundles consistent with fibrosis and tract stabilisation.
Context and relevance
Fistulating Crohn’s disease is a severe, morbid phenotype with limited effective therapies; many treatments target inflammation but not the structural stromal programmes that create and stabilise tracts. This work reframes fistula biology: rather than only immune-driven epithelial damage, specialised stromal niches—driven by developmental TFs and morphogen programmes—actively shape invasion, epithelial regeneration attempts and fibrotic stabilisation. For clinicians and translational researchers this points to stromal programmes (TWIST1/OSR2, WNT/PCP, ECM remodelling enzymes) as candidate targets to prevent tract formation or encourage resolution without compromising repair. The dataset is a rich resource for hypothesis testing and drug-target prioritisation.
Why should I read this?
Short version — read it if you care about why some Crohn’s ulcers turn into messy, persistent fistula tracts while others heal. The paper does the heavy lifting: spatially maps the cells, names the fibroblasts doing the dirty work (FAS), shows which genes and pathways make tracts invasive or fibrotic, and even tests two key transcription factors in primary cells. If you’re into translational targets or want a data-rich atlas for follow-up, this saves you months of digging.
Author style
Punchy: this is a high-impact, mechanistic spatial atlas that shifts attention from purely immune-centred views of fistulae to stromal niches as central drivers. For researchers and clinicians it’s a must-read—both practically (new targets and markers) and conceptually (developmental programmes reactivated in disease).