A skin-permeable polymer for non-invasive transdermal insulin delivery
Article Date: 19 November 2025
Article URL: https://www.nature.com/articles/s41586-025-09729-x
Article Image:
Summary
Researchers report a pH-responsive polymer (OP) that becomes skin-permeable and enables non-invasive transdermal delivery of insulin. OP is protonated (cationic) at the acidic outer stratum corneum (SC) surface, which enriches it in SC lipids, and converts to a polyzwitterion in deeper, near-neutral layers, freeing it to diffuse through the intercorneocyte lipid matrix. When insulin is covalently conjugated to OP (OP–I), the conjugate retains insulin’s receptor affinity and signalling, reaches systemic circulation via lymphatic uptake, and produces rapid, robust and prolonged hypoglycaemic effects in diabetic mice and minipigs comparable to or better than subcutaneous injection. The approach is non‑irritative in tested models and is supported by imaging, molecular dynamics simulations and mechanistic assays showing membrane-hopping and intercellular transfer in viable epidermis.
Key Points
- OP is a small (~4.5 kDa) pH-responsive polymer: cationic at skin surface pH (~5) and zwitterionic at neutral pH.
- At acidic skin surface OP binds and concentrates in SC lipids; deeper layers flip to a zwitterionic state permitting free diffusion through intercorneocyte lipids.
- Insulin conjugated to OP (OP–I) preserves insulin’s receptor binding kinetics and downstream signalling.
- Topical OP and OP–I permeate mouse and porcine (minipig) skin, enter lymphatics and reach systemic circulation within hours.
- In STZ diabetic mice and minipigs, topical OP–I lowers blood glucose rapidly and sustains normoglycaemia for ~12 hours — outperforming topical insulin or PEG–insulin controls.
- MD simulations and PMF analyses show OP/OP–I bind SC lipids under acidic conditions but have lower friction coefficients and faster lateral diffusion than insulin, explaining reduced local trapping and better permeation.
- Transport in the viable epidermis occurs via membrane-hopping and direct cell–cell transfer (cell-contact-dependent), not classical intracellular uptake — limiting intracellular degradation.
- Repeated topical application showed no skin irritation, no structural damage to SC, and no adverse systemic biochemical effects in the tested models.
- Conjugation (not mixing) of OP with cargo is required for transdermal delivery — simple co-application does not work.
- OP may be a versatile platform for transdermal delivery of other biomacromolecules (peptides, proteins, nucleic acids) pending further studies.
Content summary
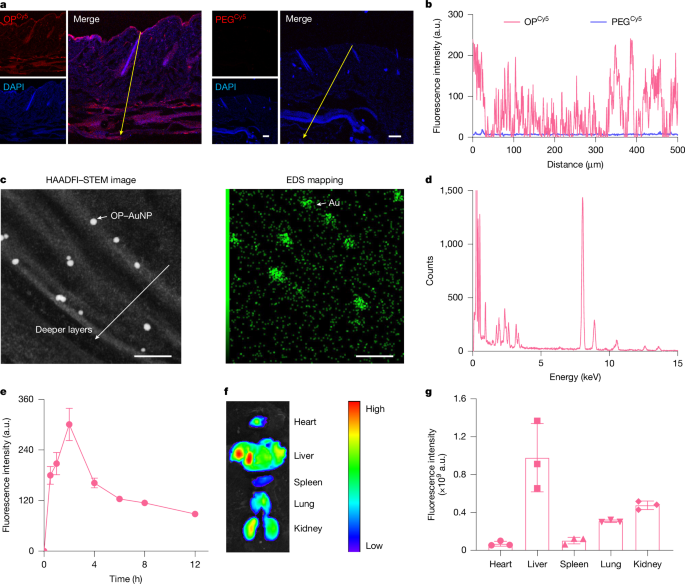
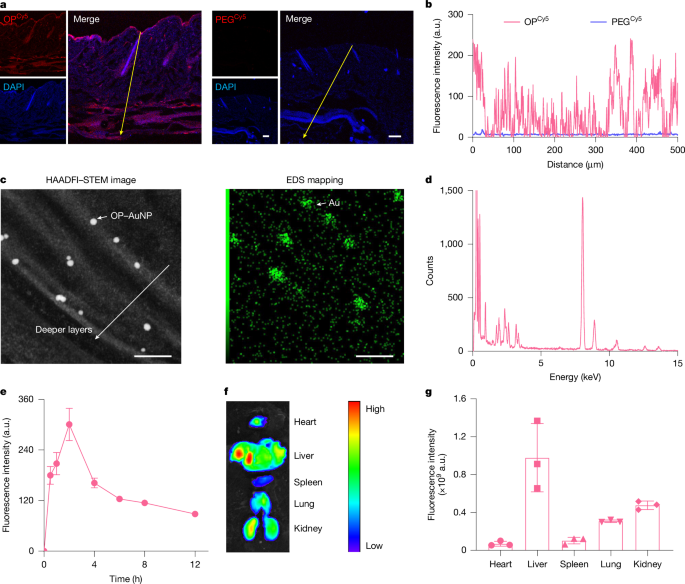
The paper introduces OP, a carefully synthesised poly(tertiary amine-oxide) that matches the skin’s pH gradient: protonated and skin-binding at pH ~5, then neutral/zwitterionic at deeper epidermal pH, enabling it to ‘stick’ then ‘slip’ through the stratum corneum lipid matrix. The team controlled OP chain length (~4.5 kDa), labelled it for imaging and tethered 5 nm gold particles to visualise intercorneocyte localisation. OP and OP–I distributed through all skin layers in mice and minipigs, and OP entered systemic circulation quickly.
Biophysical characterisation (SPR, CD, MALDI, RP‑HPLC) showed OP–I retains insulin structure and receptor affinity. In vitro 3D epidermal equivalents, intravital two-photon microscopy and HAADF‑STEM/EDS confirmed SC lipid localisation and transit. Pharmacodynamics in STZ-induced diabetic mice and diabetic minipigs showed dose-dependent, reproducible hypoglycaemia after topical OP–I cream application, sustained for hours and comparable or superior to injected insulin profiles. Safety screens indicated no local irritation or systemic toxicity in the tested timeframe.
Mechanistic work combined MD simulations, PMF, FTIR and live-cell imaging: OP binds SC lipids under acidic conditions with moderate binding energy, but transitions to a state that lowers lateral diffusion barriers and avoids ‘local trapping’. In viable epidermis OP–I remains membrane-associated and spreads between cells by contact-dependent transfer (membrane-hopping), then drains into lymphatics and circulation. Conjugation is essential — merely mixing OP with insulin did not permit transdermal uptake or glycaemic control.
Context and relevance
Non-invasive delivery of macromolecules — especially insulin — is a longstanding challenge because the stratum corneum is highly impermeable. Existing device-based or microneedle strategies work but are invasive or complex. This study presents a fundamentally different chemistry-led route that exploits the skin’s pH gradient to achieve true transdermal diffusion of a protein drug. If translatable to humans, OP-based conjugation could reduce injections, improve adherence, and change chronic care for diabetes. It also aligns with broader trends favouring on-body, needle-free therapies and precision drug‑delivery platforms. Important next steps include longer-term safety, immunogenicity testing, human skin studies and translation to manufacturable formulations and regulatory pathways.
Why should I read this
Want the short version? This could be huge — a chemistry trick that lets insulin sneak through intact skin without needles. The paper backs that claim with imaging, animal studies in mice and minipigs, receptor and simulation data, and safety checks. If you care about diabetes care, drug delivery or biomedical translation, it’s well worth the 10–15 minute read to see how they did it and what still needs proving.
Author note
Punchy take: this is a significant, rigorously demonstrated advance in transdermal macromolecule delivery. The data are broad (mechanistic, in vitro, small and large animal models) and the concept — pH-triggered bind-then-diffuse — is elegant. Read the methods and figures if you want to assess translational prospects or reproduce the chemistry and assays.