iHALT unlocks liver functionality as a surrogate secondary lymphoid organ
Summary
This Nature paper describes the discovery and characterisation of inducible hepatic lymphoid tissue (iHALT) that forms in the liver during strictly hepatotropic viral infection. Using a hepatitis C-related rodent hepacivirus (RHV) model and human HCV samples, the authors show that the liver can become a generative site for germinal-centre-like B cell activity, producing virus-specific IgG-secreting plasma cells locally while classical secondary lymphoid organs (SLOs) remain dormant.
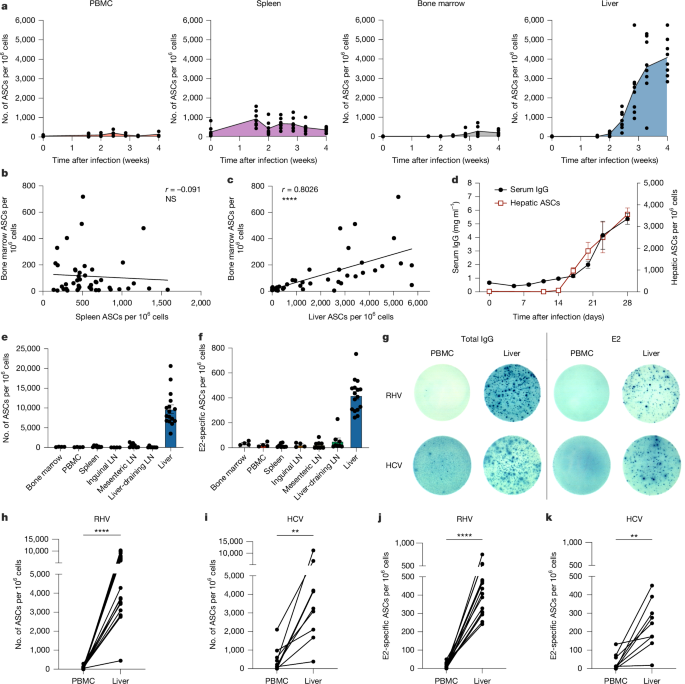
The work combines ELISpot, flow cytometry, spatial transcriptomics and B cell receptor sequencing to demonstrate that iHALT structures: generate GC-like B cells and Tfh cells in situ; produce oligoclonal, somatically hypermutated plasma cell outputs; retain plasma cells locally through validated stromal anchoring molecules (CXCL12, osteopontin, fibronectin, ICAM2 and others); and are functionally important for clearing hepaciviral infection. Human HCV liver samples show similar periportal aggregates, supporting translational relevance.
Key Points
- Strictly hepatotropic infection (RHV/HCV) induces local hepatic lymphoid structures (iHALT) that generate virus-specific antibody responses within the liver.
- iHALT arises while SLOs (spleen, lymph nodes) show minimal GC activity — iHALT compensates for SLO dormancy.
- Intrahepatic IgG+ antibody-secreting cells (ASCs) and E2-specific plasma cells expand massively by week 4 in mice and are enriched in human HCV liver samples.
- Local GC-like B cells, T follicular helper (Tfh) cells and plasma cells are present in periportal foci and show markers of proliferation and somatic hypermutation.
- Plasma cells are retained adjacent to iHALT by validated adhesion/chemokine pairs (VLA-4, LFA-1, CXCR4 with fibronectin, ICAM2, CXCL12, osteopontin); disrupting these reduces intrahepatic ASCs.
- iHALT structures are anatomically oligoclonal (few dominant B cell clones) yet achieve SHM levels comparable to SLO GCs.
- Functional experiments (splenectomy, FTY720 treatment, CD40L blockade, BCR-restricted mice) show iHALT-dependent humoral responses are antigen- and T cell-dependent and necessary for viral clearance.
- Spatial transcriptomics and single-cell-resolution Xenium data show remarkable cellular and organisational similarity between mouse iHALT and human HCV-associated intrahepatic lymphoid aggregates.
Content summary
Secondary lymphoid organs are the canonical sites for germinal-centre (GC) reactions that produce high-affinity, class-switched antibody responses. This study shows that, during strictly hepatotropic infection, the liver itself forms inducible lymphoid hubs (iHALT) that support local GC-like activity and plasma cell output while SLOs remain largely inactive.
In RHV-infected mice, intrahepatic ASCs expand hundreds-fold and correlate with serum IgG rises. Blocking lymphocyte egress from SLOs or removing the spleen does not prevent viral clearance, indicating local hepatic responses suffice. By contrast, systemic infection (LCMV) relies on SLO GC responses with trafficking of ASCs to tissues.
iHALT sites show intermingled B and T cell populations rather than the strict compartmentalisation seen in lymph nodes. iHALT B cells display GC signatures (proliferation, DNA repair and Aicda expression), produce somatically hypermutated antibodies and give rise to plasma cells that are retained periportally via chemokine/adhesion interactions. Disrupting retention molecules disperses intrahepatic plasma cells and impairs the local ASC pool.
Human liver samples from chronic HCV patients contain similar periportal generative foci and plasma cell niches, supporting the model’s human relevance. The authors propose iHALT is a bona fide inducible lymphoid tissue that can compensate for SLO suppression during hepatotropic infection and materially affects infection outcome.
Context and relevance
This work reframes how we understand organised humoral immunity at organ sites of infection. Instead of being wholly dependent on distant SLO-generated responses, the liver can host local, functional germinal-centre-like activity that yields retained antibody-secreting cells. That matters for hepatitis virus biology, persistence versus clearance, vaccine strategies targeting hepatic pathogens and for interpreting intrahepatic immune aggregates in liver disease and cancer.
For immunologists and clinicians, the findings highlight new mechanisms of local immunity, potential stromal targets to modulate tissue-resident plasma cells, and reasons why some hepatic infections clear while others persist. For translational researchers, iHALT suggests opportunities to steer local B cell responses or disrupt pathogenic local antibody production in autoimmunity.
Why should I read this?
Because this paper does something neat: it shows the liver can act like a pop-up lymph node that actually makes useful, virus-killing antibodies in place. If you work on hepatitis viruses, tissue immunity, vaccines or tissue-resident plasma cells — this saves you poking through a lot of scattered studies. It’s a well-controlled mouse model backed up by human tissue data, and it points to concrete molecules that hold plasma cells in the liver. Handy if you want new angles on stopping persistence or tweaking local immunity.
Source
Article Date: 26 November 2025