Inhibitory PD-1 axis maintains high-avidity stem-like CD8+ T cells
Summary
This study uses high-dimensional 3D imaging and conventional immunology to map how tumour-draining lymph nodes (tdLNs) maintain stem-like CD8+ T (TSL) cells. The authors show that PD-1 and its ligands (PD-L1/PD-L2), engaged in microclusters at T cell–dendritic cell synapses, attenuate TCR signalling in high‑affinity clones. That attenuation preserves a pool of high‑avidity, TCF-1+SLAMF6+ stem‑like CD8+ T cells in cDC1-rich niches of the tdLN. Late antigen presentation by cDC1 cells drives selective expansion and affinity evolution of those TSL clones. Broad PD-1/PD-L1/PD-L2 blockade provokes proliferation and effector differentiation but also reduces numbers and average TCR affinity of the SLAMF6+ TSL pool (via differentiation and activation‑induced death), producing a reshaped clonal repertoire with potential long‑term consequences for anti‑tumour immunity.
Key Points
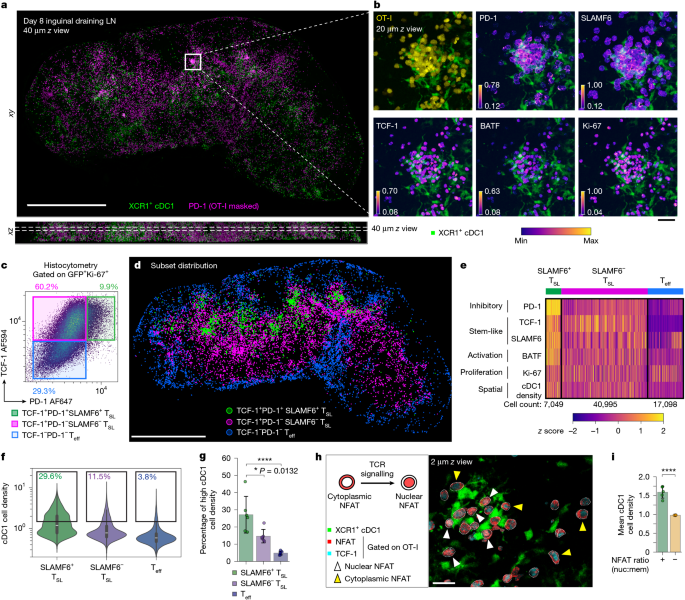
- High‑resolution 3D imaging revealed clusters of PD‑1+TCF‑1+SLAMF6+ CD8+ T cells tightly associated with XCR1+ cDC1 cells in tdLNs.
- These SLAMF6+ TSL cells show ongoing TCR signalling (nuclear NFAT) yet retain stem‑like features due to PD‑1 engagement at the synapse.
- Late antigen presentation by cDC1 in tdLNs is required to expand and enrich high‑affinity TSL clones over time.
- PD‑1/PD‑L1/PD‑L2 signalling attenuates TCR input and preserves stemness of high‑avidity clones; blocking this axis causes TCF‑1/SLAMF6 downregulation and increased apoptosis among those cells.
- Checkpoint blockade (especially dual PD‑L1/PD‑L2) increases immediate effector expansion and tumour control but reduces the number and mean TCR affinity of SLAMF6+ TSL cells, shifting clonal competition toward lower‑affinity clones.
- Partial or single‑ligand blockade can preserve high‑affinity TSL cells but at the cost of weaker anti‑tumour responses in the short term.
Content summary
Using adoptive transfer models and polyclonal antigen responses, the team imaged day‑8 tdLNs with a multiplex Ce3D 3D pipeline and analysed >1 million single‑cell events. They defined TSL (TCF‑1+) subsets and found a PD‑1hiSLAMF6hi fraction localised in cDC1‑dense niches and actively signalling (nuclear NFAT). Ablation of cDC1 during this late phase reduced expansion of SLAMF6+ TSL cells, showing these dendritic cells are necessary to sustain late antigen‑driven TSL proliferation and affinity maturation. Analyses using tetramer:CD3 ratios as an imputed TCR affinity index showed higher‑affinity clones were enriched among PD‑1hi SLAMF6+ TSL cells and that average affinity increased between day 6 and day 13. Imaging of PD‑1 and PD‑L1 co‑localisation revealed PD‑1 polarisation at synapses; functional blockade of PD‑1 ligands triggered rapid proliferation but loss of TCF‑1/SLAMF6 expression and increased cleaved caspase‑3 in high‑affinity TSL cells. Longitudinal and modelling data indicate that intense PD‑1 pathway blockade can permanently deplete high‑affinity TSL clones, while partial blockade may avoid that depletion but delivers less tumour control per treatment cycle.
Context and relevance
These results change how we think about PD‑1 in antitumour responses: rather than simply suppressing useful cells, PD‑1 here enables high‑affinity clones to persist as stem‑like progenitors by damping excessive TCR signals during continued antigen exposure in tdLNs. The work links spatial dendritic cell niches to affinity selection and reveals a trade‑off in checkpoint therapy — strong blockade expands effectors but may erode the very high‑avidity progenitors needed for durable responses. That has clear implications for immunotherapy dosing, timing and for strategies combining checkpoint blockade with vaccines, adoptive cell transfer or dendritic cell‑targeted approaches.
Author style
Punchy: this paper is a tidy, mechanistic tour de force. It demonstrates that PD‑1 is not just a brake that limits responses — it’s a tuner that preserves the best T cell clones as stem‑like reserves. If you care about how checkpoint therapy reshapes TCR repertoires and long‑term immunity, read the data closely.
Why should I read this?
Short answer: because it flips a common assumption. Want to know why some checkpoint treatments give great short‑term tumour shrinkage but variable long‑term benefit? This paper shows PD‑1 signalling helps protect high‑affinity progenitor T cells in lymph nodes — and that stripping away that protection can boost immediate killing yet lose those top‑quality clones for good. Handy if you design or interpret immunotherapy trials, or if you’re thinking about dosing/timing tweaks.