Human gut M cells resemble dendritic cells and present gluten antigen
Summary
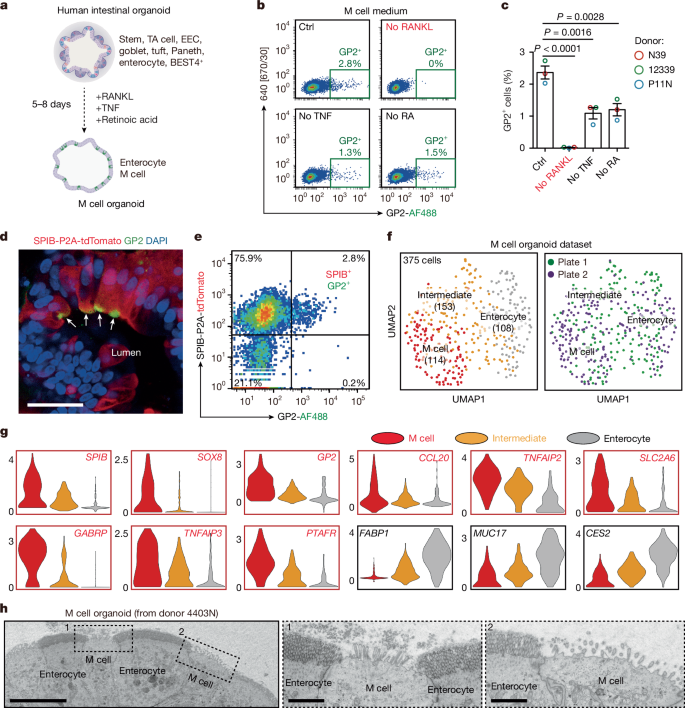
This Nature paper reports that human intestinal microfold (M) cells — a rare epithelial cell type that samples luminal antigens — share transcriptional programmes and functional features with dendritic cells and can act as non-haematopoietic MHC-II antigen-presenting cells. Using human intestinal organoid models adapted to generate M cells, the authors define M cell differentiation stages (SPIB+ICAM2− early, SPIB+ICAM2+ immature, SPIB+ICAM2+GP2+ mature), identify ICAM2 as an M-lineage marker, and show that human M cells constitutively express MHC-II and contain MIIC compartments for peptide loading.
The study demonstrates functional antigen presentation: M cells from HLA-DQ2.5 donors take up gliadin (wheat) peptides, load immunogenic gliadin:HLA-DQ2.5 complexes and activate gluten-specific CD4+ T cells. M cells highly express tissue transglutaminase (TGM2), which deamidates gliadin peptides into the pathogenic form required for strong T cell recognition. Key regulators of human M cell differentiation include SPIB and RUNX2, and CSF2 promotes M cell maturation. Importantly, many of these dendritic-cell-like features are human-specific and not observed in mouse M cells.
Key Points
- Human M cells can be generated in organoids with RANKL, TNF and retinoic acid; these organoids recapitulate M cell morphology and markers (GP2, SPIB, SOX8).
- ICAM2 marks early/immature M-lineage cells and, together with SPIB and GP2, defines a clear human M cell differentiation trajectory.
- Human M cells express MHC-II and form MIIC compartments, enabling antigen processing and peptide loading — features resembling dendritic cells.
- M cells take up gliadin, present gliadin:HLA‑DQ2.5 complexes and directly activate gluten-specific CD4+ T cells from coeliac patients in co-culture assays.
- M cells express high levels of TGM2 and can deamidate gliadin peptides (Q→E), producing the pathogenic epitopes required for strong HLA‑DQ2.5-restricted T cell activation.
- Transcription factors SPIB and RUNX2 are essential for human M cell development; CSF2 enhances maturation — some regulatory programmes are shared with dendritic cells.
- These antigen‑presentation features are largely human specific; mouse M cells lack constitutive MHC-II expression, underscoring species differences for translational studies.
Why should I read this?
Short version: if you care about how the gut talks to the immune system — and especially how gluten triggers coeliac disease — this paper changes the story. Human M cells aren’t just sampling ports: they can present antigen and even make the nasty, disease-triggering gluten pieces. It’s neat, unexpected and directly relevant if you work on gut immunity, coeliac disease or organoid models.
Context and relevance
This work reframes M cells as professional, non-haematopoietic antigen-presenting cells in human gut-associated lymphoid tissue. It links M cell biology to coeliac disease pathogenesis by showing M cell uptake, processing (including TGM2-mediated deamidation) and HLA‑DQ2.5-restricted presentation of gliadin peptides that activate pathogenic CD4+ T cells. The finding that many antigen‑presentation traits are absent in mouse M cells highlights a crucial species difference and warns against over-reliance on murine models for certain aspects of human intestinal immunity. The organoid co-culture assays also provide a tractable human platform for mechanistic and therapeutic investigations into coeliac disease and mucosal antigen presentation.
Author style
Punchy: this is a significant, potentially paradigm-shifting study. It elevates M cells from passive samplers to active participants in antigen presentation and provides mechanistic links to coeliac disease — a must-read for researchers in mucosal immunology and translational gut research.