Pancreatic cancer is evasive. Is the nervous system the reason why?
Article Date: 2025-12-10
Article URL: https://www.nature.com/articles/d41586-025-03943-3
Article Image: 
Summary
This Nature feature explores a rapidly growing field — cancer neuroscience — and how pancreatic ductal adenocarcinoma (PDAC) exploits the nervous system to survive, grow and evade treatment. The story begins with an unexpected finding: newborn mice treated with capsaicin (which blocks sensory nerves) did not develop pancreatic tumours, suggesting nerves play a direct role in tumour formation.
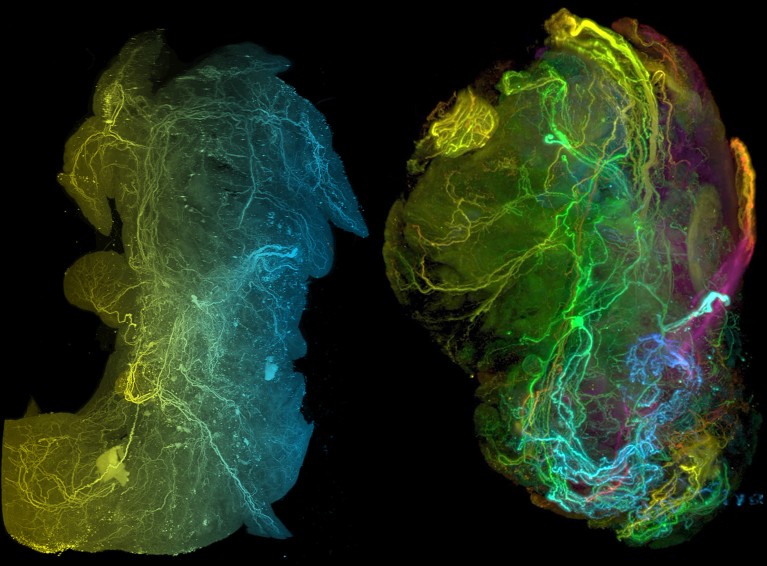
Researchers have since uncovered multiple mechanisms by which PDAC recruits, reprogrammes and even mimics neurons: tumours secrete nerve growth factors to attract axons; cancer cells form pseudo-synapse-like contacts that supply growth-promoting neurotransmitters; tumours rely on neuron-supplied metabolites such as serine; and tumours co-opt neuronal immune-suppressive tactics (the “safe harbour” idea) that may blunt immunotherapy.
The piece covers new techniques (for example, Trace-n-Seq to trace and sequence tumour-associated neurons), evidence that tumours change neuronal gene expression, and early translational strategies combining nerve-targeting with immunotherapy or radiation to improve immune infiltration and treatment response.
Key Points
- A serendipitous mouse experiment with capsaicin suggested sensory nerves are essential to PDAC development and progression.
- PDAC secretes nerve growth factors and axonal guidance molecules to attract and sustain tumour-associated nerves.
- Cancer cells form pseudo-synapses with neurons, using neurotransmitters (eg glutamate) and neuron-derived metabolites (eg serine) to fuel growth.
- Neurons connected to tumours show altered gene expression, implying the tumour reprogrammes the nervous microenvironment (Trace-n-Seq revealed these differences).
- Pancreatic tumours may exploit neuronal immune-suppressive mechanisms (checkpoint proteins on neurons), helping explain poor immunotherapy responses.
- Preclinical combos — killing or irradiating tumour-associated nerves alongside immunotherapy — improved tumour control in mice and are being prepared for clinical testing.
Context and relevance
Pancreatic cancer is one of the deadliest cancers, with low five-year survival. This article ties together decades-old observations of neural invasion with modern molecular tools to show the nervous system is not just a bystander but an active participant in PDAC biology. The findings intersect oncology, neuroscience and immunology, and point to new therapeutic avenues: rather than replacing existing therapies, nerve-targeting strategies may sensitize tumours to chemo- and immunotherapies.
For clinicians and researchers, the work reframes PDAC as a disease that co-opts physiological neuronal support systems (growth factors, metabolite supply, immune modulation). For drug development, it highlights nervous-system-derived targets (neurotransmitter signalling, nerve growth-factor pathways, neuronal checkpoint interactions) that could be combined with current treatments.
Why should I read this?
Short and blunt: if you care about why pancreatic cancer is so stubborn and how researchers might finally start to shift outcomes, this piece is gold. It bundles surprising animal findings, new single-cell and tracing technologies, and practical ideas for combination therapies — all in one place. We’ve sifted the science so you don’t have to: read this to get the big, actionable picture fast.