Lesion-remote astrocytes govern microglia-mediated white matter repair
Article Date: 17 December 2025
Article URL: https://www.nature.com/articles/s41586-025-09887-y
Article Image: Figure 1
Summary
This Nature study maps how spared (lesion-remote) astrocytes (LRAs) in the injured spinal cord adopt distinct, region-specific reactive states and orchestrate local microglial responses that are essential for white matter debris clearance and functional recovery.
Key Points
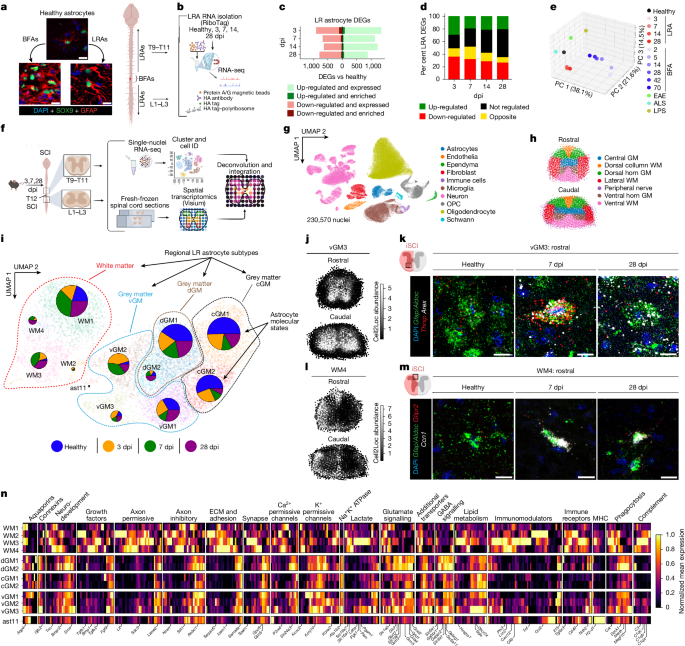
- Lesion-remote astrocytes (LRAs) acquire molecularly distinct, spatiotemporally evolving reactivity states that differ from border-forming astrocytes (BFAs) and other injury models.
- A white-matter LRA subtype selectively upregulates the matricellular protein CCN1 in response to local myelin degeneration.
- CCN1 from LRAs colocalises with phagocytic microglia nodules (white matter degeneration-associated microglia, WDM) and modulates their transcriptional profile toward repair-associated, lipid-handling states.
- Astrocyte-specific deletion of Ccn1 (Ccn1-cKO) causes exaggerated accumulation of debris-engorged microglial nodules, but paradoxically impairs efficient myelin/axon debris clearance and lipid storage (fewer lipid droplets, altered lipidome).
- Loss of astrocyte CCN1 impairs recovery of specific sensory modalities (cold thermoception) after incomplete spinal cord injury while locomotor recovery remains intact.
- CCN1 acts on microglia via a CCN1–SDC4 axis to promote lipid uptake/storage and to suppress cholesterol efflux, thereby facilitating intracellular digestion of myelin-derived lipids.
- CCN1+ LRAs are induced by myelin damage (not by axon degeneration alone) and are observed in other demyelinating models (LPC, EAE) and in human MS and SCI white matter, indicating evolutionary and disease relevance.
Content summary
The authors used RiboTag bulk RNA-seq, single-nucleus RNA-seq and spatial transcriptomics across multiple post-injury time points after anatomically incomplete spinal cord injury to define 12 astrocyte molecular states. LRAs in spared regions show marked transcriptional reprogramming that is largely distinct from BFAs and from astrocyte states in non-traumatic disorders. In the white matter, LRAs progress through WM2/WM3 to a chronic WM4 state characterised by metabolic gene programmes and immune-modulatory transcripts.
Deconvolution of spatial data uncovered an ipsilesional white matter niche where CCN1-expressing astrocytes neighbour WDM microglia nodules. Functional experiments with astrocyte-selective Ccn1 knockout mice show accelerated, spatially aberrant microglial nodule formation, increased intracellular myelin/axon debris burden but impaired extracellular debris clearance and fewer microglial lipid droplets. Behavioural assays reveal selective deficits in cold-sensing recovery in Ccn1-cKO mice.
In vitro and in vivo mechanistic work demonstrates that recombinant CCN1 directly reprogrammes primary microglia toward WAM/DAM-like transcriptional programmes (upregulating lipid storage genes and lowering efflux-related genes). Proteomics and blocking antibodies implicate SDC4 as a CCN1 receptor on microglia; blocking SDC4 prevents CCN1-driven lipid droplet accumulation. Lipidomics of microglia from injured Ccn1-cKO spinal cords reveal accumulation of myelin-associated lipid species and reduced cholesterol ester/triacylglycerol storage, supporting a failure of adaptive lipid buffering.
Context and relevance
This paper defines LRAs as active, region-specific organisers of post-injury white matter inflammation and repair rather than passive bystanders. It links a precise astrocyte-secreted ligand (CCN1) to microglial lipid-handling machinery and functional recovery, highlighting astrocyte→microglia signalling as a potential therapeutic axis for disorders with myelin loss (trauma, MS, demyelinating diseases).
Given growing recognition that impaired lipid metabolism in phagocytes limits remyelination and repair, the CCN1–SDC4 pathway is a notable candidate for strategies aimed at improving debris clearance and limiting chronic white matter inflammation.
Author style
Punchy: this is a big, well-mapped piece of work that moves astrocytes from passive markers of injury to active conductors of microglial lipid metabolism and repair. If you work on CNS repair, glia interactions or remyelination, the mechanistic detail here — CCN1, SDC4, lipid droplet biology — is directly actionable and worth digging into.
Why should I read this?
Short answer: because it explains how astrocytes in spared tissue actually help (or hinder) white matter clean-up after injury. Want to know why some microglia get stuffed with myelin and stall? This paper shows astrocyte CCN1 helps microglia stash and process lipid debris so repair can happen — and without it you get more inflammation but worse clearance and sensory recovery. It’s neat, mechanistic and relevant if you care about remyelination or post-injury inflammation.