Transient hepatic reconstitution of trophic factors enhances aged immunity
Article Date: 17 December 2025
Article URL: https://www.nature.com/articles/s41586-025-09873-4
Article Image:
Summary
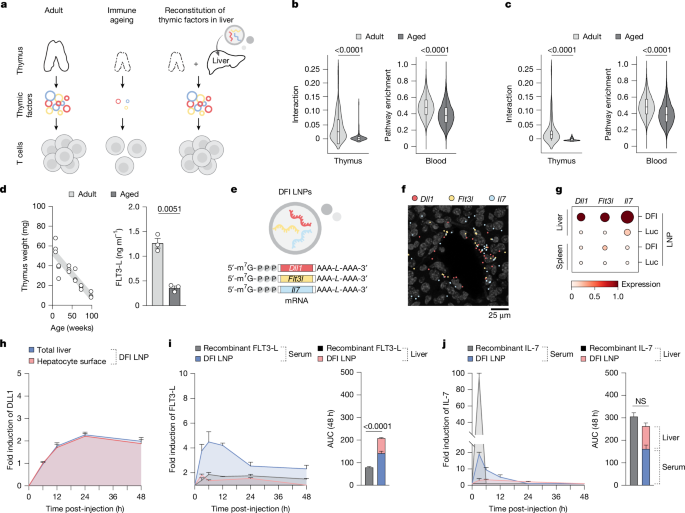
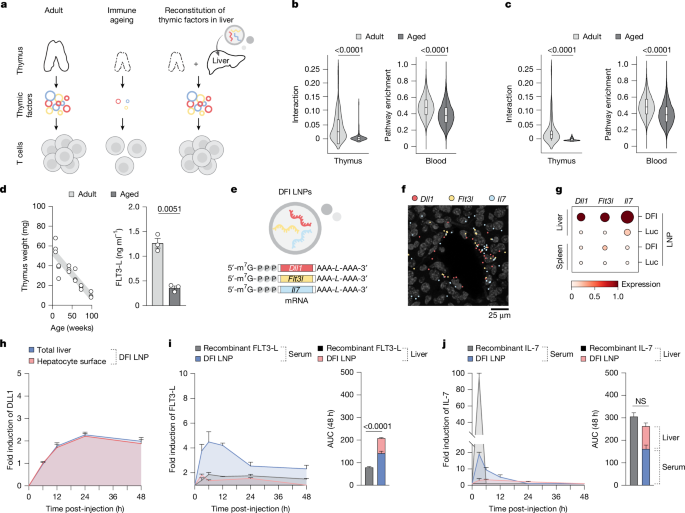
This Nature paper reports a clever, disease-agnostic strategy to counter immune ageing by turning the liver into a short-term factory for thymus-derived trophic cues. The authors packaged mRNAs encoding DLL1 (Notch ligand), FLT3‑L and IL‑7 (the DFI combo) into lipid nanoparticles (SM-102 LNPs) and delivered them systemically to aged mice. The mRNAs translated primarily in hepatocytes, producing surface DLL1 and secreted FLT3‑L and IL‑7 that circulated or remained matrix-associated in the liver for hours to days.
The transient hepatic production of these factors restored several age-related immune defects: increased common lymphoid progenitors (CLPs), boosted thymopoiesis and recent thymic emigrants, expanded naive CD4+ and CD8+ T cell pools, improved dendritic cell and B cell composition, enhanced vaccine responses (OVA model) and revitalised anti-tumour immunity — including synergy with anti‑PDL1 checkpoint blockade. Importantly, repeated dosing showed minimal liver toxicity, no evidence of systemic autoimmunity in multiple models (NOD, Act‑mOVA, EAE), and the effects waned after treatment stopped, consistent with the transient nature of mRNA delivery.
Author style: Punchy — this is a high-impact, translationally minded study that shows a neat way to restore systemic immune signals without invasive thymic surgery or chronic cytokine infusions.
Key Points
- Ageing reduces thymic trophic signals (Notch, IL‑7, FLT3‑L) and contracts naive T cell output; the liver remains protein‑synthesis competent in old age.
- Authors delivered m1Ψ‑modified mRNAs for DLL1, FLT3‑L and IL‑7 (DFI) in SM‑102 LNPs, leading to hepatocyte surface DLL1 and secreted IL‑7 and FLT3‑L with sustained, controllable kinetics.
- DFI increased bone marrow CLPs, CCR9+ progenitors that home to the thymus, and restored thymocyte maturation and thymic cellularity in aged mice, raising peripheral naive T cell counts without increasing clonal dominance.
- DFI improved vaccine-induced CD8+ responses and cytokine recall (IL‑2, IFNγ) in aged mice; the full DFI combo was required for the broad benefit, single factors had narrower effects.
- Preconditioning with DFI enhanced anti‑tumour immunity and synergised with anti‑PDL1 in aged tumour models, increasing tumour-specific CD8+ TILs and repertoire diversity.
- DFI treatment showed limited toxicity (no major liver function changes, minimal histological inflammation) and did not break tolerance or accelerate autoimmunity in NOD, Act‑mOVA or EAE models.
- The immune benefits are transient and largely confined to the dosing window, implying repeated dosing would be necessary for durable effect; long‑term safety remains to be established.
Why should I read this?
Want a neat, less invasive strategy to boost immunity in older adults without permanent surgery or constant high‑dose cytokines? This paper shows a clever shortcut: use the liver as a temporary factory to top up the exact signals that fall with age. It’s full of practical data — from single‑cell maps to vaccine and tumour models — so if you care about ageing, immunotherapy or mRNA therapeutics, it saves you the slog of reading a huge methods section yourself.
Context and relevance
This study sits at the intersection of immunosenescence research and mRNA therapeutics. It addresses a core problem — thymic involution and loss of trophic cues — by repopulating those signals extracorporeally. The approach complements other rejuvenation strategies (HSC manipulation, thymic regeneration) with a technically simpler, scalable route that leverages recent advances in mRNA‑LNP delivery. Clinically, it points to potential adjuvant use for vaccines and checkpoint immunotherapies in older patients, but translation will need careful long‑term safety work and dose scheduling studies.