Dogma-defying signalling through G proteins could lead to better pain relief
Summary
New work discussed by Andrew B. Tobin highlights surprising behaviour in G proteins that challenges long-standing assumptions about G-protein signalling. Two recent papers (one in Nature by Stahl et al. and a companion in Nature Communications) describe GTP-release-selective agonism at G-protein-coupled receptors (GPCRs) that prolongs opioid analgesic efficacy. The findings suggest a new mechanism by which agonists can bias G-protein function, potentially enabling the design of opioid drugs that deliver stronger, longer-lasting pain relief with fewer adverse effects.
Key Points
- Researchers discovered GTP-release-selective agonists that change how G proteins signal after receptor activation.
- These agonists can prolong opioid analgesic effects, offering longer pain relief in preclinical models.
- The work overturns parts of the prevailing dogma about uniform G-protein activation and nucleotide exchange dynamics.
- Mechanistic insights come from complementary studies published in Nature and Nature Communications.
- Implication: rational design of next-generation opioids that separate analgesia from common side effects may be feasible.
Content summary
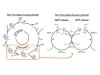
The commentary explains that decades of G-protein research did not anticipate the specific way some agonists can selectively affect GTP release from G proteins. Stahl et al. report agonists that favour a state in which G proteins are activated but release GTP more slowly or selectively, altering downstream signalling duration and profile. A companion study characterises the GTPγS release function and complements the mechanistic picture.
Tobin outlines why these mechanistic twists matter: by changing the timing and nature of G-protein actions at opioid receptors, drug developers might design compounds that sustain analgesia without triggering the same cascade of responses tied to tolerance or other adverse effects. The commentary places the new findings in the context of GPCR structural biology and biased signalling, and highlights the translational potential for pain therapeutics.
Context and relevance
This is important for anyone following drug discovery or neuropharmacology. GPCRs are the target of many medicines, and opioids remain a central clinical tool with serious limitations. Discovering a controllable, mechanistic handle on G-protein activity offers a fresh route to improve efficacy and safety profiles of analgesics. These findings connect to wider trends in biased agonism and precision-targeted receptor pharmacology.
Why should I read this?
Quick and blunt: if you care about better pain drugs (patients, researchers, pharma people), this is where the next clever tweaks might come from. Tobin sums up two papers that could change how we design opioids — saving you the time of wading through dense methods and showing the real-world payoff: longer, stronger pain relief with fewer nasties.
Author style
Punchy and to the point — Tobin highlights the significance without overclaiming. The piece frames the new studies as a notable paradigm nudge with clear translational promise.