Albumin orchestrates a natural host defence mechanism against mucormycosis
Summary
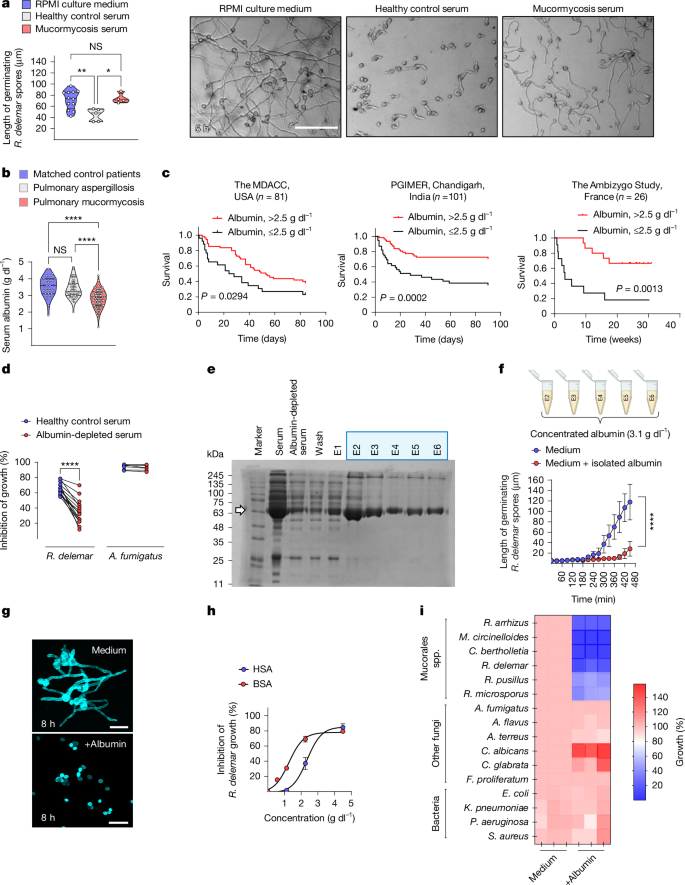
This Nature study shows that human serum albumin (HSA) is a specialised host defence factor that selectively inhibits growth and pathogenicity of Mucorales fungi—the agents of mucormycosis. The antifungal activity is mediated by albumin-bound physiological free fatty acids (FFAs) (notably caprylic acid and others) which are delivered to fungal spores, block filamentous growth, inhibit protein synthesis and suppress key virulence factors such as mucoricin and the CotH3 invasin. Severe hypoalbuminaemia, glycation or oxidative modification of FFAs impairs this defence and is associated with poorer outcomes in patients. Albumin-deficient mice are highly susceptible to mucormycosis; albumin supplementation restores resistance.
Key Points
- Human serum albumin (HSA) at physiological concentrations selectively inhibits hyphal growth of Mucorales but not other major fungal or bacterial pathogens.
- The active agents are albumin-bound free fatty acids (FFAs); caprylic acid (C8:0) and a range of medium/long-chain FFAs exhibit potent anti‑Mucorales activity in physiological ranges.
- Albumin protects FFAs from oxidation and shuttles them to fungal spores; oxidised FFAs lose uptake and antifungal potency.
- Albumin-bound FFAs arrest fungal protein synthesis, downregulate virulence genes (mucoricin, CotH3) and prevent tissue necrosis and invasion in mouse lungs.
- Severe hypoalbuminaemia and albumin glycation (as in poorly controlled diabetes) correlate with loss of serum anti‑Mucorales activity and worse clinical outcomes; albumin replacement rescued resistance in albumin‑KO mice.
Content summary
Mucormycosis is a rapidly invasive fungal infection with high mortality. The authors compared sera from healthy donors and patients with mucormycosis and found that the latter lose anti‑Mucorales activity and commonly have low albumin. Depleting albumin from healthy serum abolishes its inhibitory effect against Rhizopus delemar; purified albumin or albumin filtrate (containing bound ligands) reproduces the effect.
Lipid fractionation and lipidomics identified caprylic acid and multiple physiological FFAs in albumin filtrates as the inhibitory molecules. Charcoal-stripped (FFA‑free) albumin has no activity; reloading albumin with FFAs restores it. Oxidation or glycation of albumin/FFAs reduces FFA binding, increases FFA oxidation and eliminates anti‑fungal action.
Mechanistically, albumin‑bound FFAs accumulate in germinating spores via active uptake, rapidly inhibit global translation (OP‑puro assay) and suppress expression of virulence factors including mucoricin and CotH3. Pre‑treating spores with albumin or FFAs renders them avirulent in immunocompetent mice, preventing tissue necrosis and reducing fungal burden. Albumin knockout humanised mice are selectively susceptible to mucormycosis; administration of FFA‑free HSA restores resistance.
Context and relevance
This work reveals a previously unrecognised immunometabolic mechanism: albumin acts as a carrier and protector of physiological FFAs that function as host antimicrobial effectors against Mucorales. It connects clinical risk factors—hypoalbuminaemia, diabetes‑related albumin glycation, oxidative stress and malnutrition—with molecular loss of defence, offering a plausible explanation for the epidemiology of mucormycosis in metabolically unwell patients and COVID‑19 associated cases.
Clinical implications include using serum albumin as an accessible prognostic biomarker and exploring albumin correction or targeted FFA therapies as low‑cost preventive or adjunctive measures in high‑risk patients. The mechanistic insight (protein synthesis arrest and virulence suppression) gives a clear target for future therapeutic development.
Why should I read this
Short answer: because it explains a major mystery — why certain metabolic conditions drastically raise mucormycosis risk — and points to simple, testable clinical fixes (check albumin, consider replacement). It’s packed with human data, elegant biochemistry and solid mouse validation, so you won’t waste time hunting down the story yourself.
Author style
Punchy: this is a clear, high‑impact finding that reframes albumin from a passive blood protein to a frontline metabolic immune effector against an often‑fatal fungal infection. If you manage high‑risk patients or work on fungal pathogenesis, the details matter.