Mitochondrial transfer from glia to neurons protects against peripheral neuropathy
Article Date: 07 January 2026
Source: Nature
Summary
This study shows that satellite glial cells (SGCs) in dorsal root ganglia (DRG) transfer mitochondria to neighbouring sensory neurons. Transfer occurs via tunnelling nanotube-like structures (TNTs), endocytosis and gap-junction pathways, is activity-dependent, and is regulated by the motor protein MYO10. Chemotherapy (paclitaxel) and diabetes impair SGC–neuron contacts, reduce mitochondrial transfer and drive peripheral nerve degeneration and pain. Crucially, adoptive transfer of healthy SGCs or isolated SGC mitochondria (including human mitochondria) into the DRG restores neuronal bioenergetics, prevents intraepidermal nerve-fibre loss and reduces neuropathic pain in mouse models — effects that require functional MYO10 and intact mitochondria.
Key Points
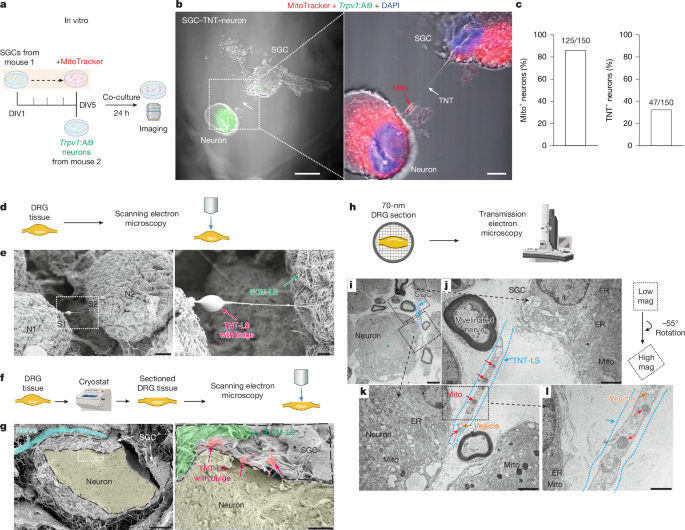
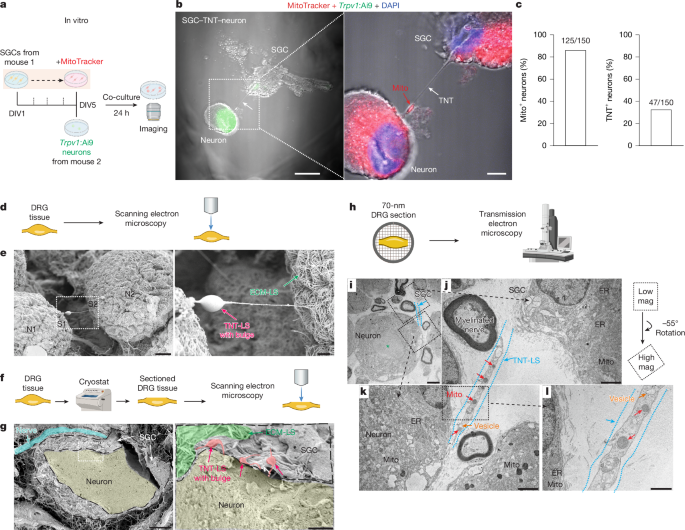
- SGCs directly donate mitochondria to DRG sensory neurons in vitro, ex vivo and in vivo; mitochondria are visible inside TNT-like tubes and by EM.
- Transfer mechanisms include TNTs, endocytosis and connexin-mediated gap junctions; neuronal activity promotes transfer.
- MYO10 is enriched in SGCs, required for TNT formation and mitochondrial transfer; reducing MYO10 increases pain sensitivity and disrupts SGC–neuron contacts.
- Pathological states (chemotherapy-induced peripheral neuropathy and diabetic neuropathy) disrupt TNTs, reduce transfer and cause mitochondrial dysfunction and intraepidermal nerve-fibre (IENF) loss.
- Therapeutic proof-of-concept: intra-DRG delivery of healthy SGCs or isolated SGC mitochondria (mouse and human) improves mitochondrial respiration, rescues IENF density and alleviates mechanical and ongoing pain in mouse models; damaged or myxothiazol-treated mitochondria do not help.
- Human DRG show TNT-like structures and high MYO10 expression in SGCs; diabetic donors have reduced MYO10 and impaired SGC→neuron mitochondrial transfer ex vivo, supporting translational relevance.
Content summary
The authors combined primary cell co-cultures, whole-mount and sectioned DRG imaging (SEM/TEM/SIM), genetic MitoTag labelling, pharmacological blockade and behavioural models to map mitochondrial transfer from SGCs to sensory neurons. In MitoTag mice with SGC-specific labelling, neuron mitochondrial labelling increased over time and after spared nerve injury; blocking TNT formation (cytochalasin B) or endocytosis (Pitstop2) reduced neuronal mitochondrial labelling. MYO10 knockdown or heterozygous loss reduced TNTs and transfer and produced mechanical/thermal hypersensitivity. Chemotherapy (paclitaxel) and diabetes led to enlarged neuron–SGC gaps, altered mitochondrial morphology and reduced transfer. Adoptive transfer of healthy SGCs or isolated mitochondria into DRG restored mitochondrial function and reduced pain measures in paclitaxel and diabetic mouse models; the effect required intact MYO10 and functional mitochondria. Single-nucleus RNA-seq and in situ hybridisation in human DRG confirm SGC enrichment of MYO10 and reduced expression in diabetes, with ex vivo human SGC–neuron co-cultures showing impaired mitochondrial donation from diabetic donors.
Context and relevance
Peripheral neuropathies (chemotherapy-induced and diabetic) are commonly linked to mitochondrial dysfunction and small-fibre degeneration. This work identifies a previously underappreciated neuro–glial supply route whereby SGCs support neuronal bioenergetics and axonal maintenance by donating mitochondria. The discovery ties a specific cellular mechanism (MYO10-driven TNTs plus endocytosis/gap-junction uptake) to neuropathic pathology and suggests two translational strategies: adoptive SGC transfer or direct mitochondrial delivery to DRG. The human DRG data strengthen clinical relevance and explain how glial dysfunction could drive small-fibre neuropathy.
Why should I read this?
Because it’s a neat, slightly mind-bending fix for a thorny problem: glia actually hand over mitochondria to keep sensory neurons alive and quiet. If you follow pain, peripheral neuropathy or mitochondrial therapies, this paper saves you the slog — it shows the mechanism, the regulator (MYO10), the disease link (chemo/diabetes) and even a potential therapy that works in mice and uses human material. Short version: glia gift mitochondria, and that matters.
Author style (punchy)
This is a high-impact, mechanistic and translational study. It re-frames SGCs from passive support cells to active mitochondrial donors that protect neurons from degeneration and pain. The therapeutic angle (cell or mitochondrial transfer) is tangible and backed by cross-species evidence — so this isn’t just a curiosity, it’s potentially practice-changing for peripheral neuropathy research.