Oral 4′-fluorouridine rescues nonhuman primates from advanced Lassa fever
Summary
Article Date: 07 January 2026
Article URL: https://www.nature.com/articles/s41586-025-09906-y
Article Image: https://media.springernature.com/lw685/springer-static/image/art%3A10.1038%2Fs41586-025-09906-y/MediaObjects/41586_2025_9906_Fig1_HTML.png
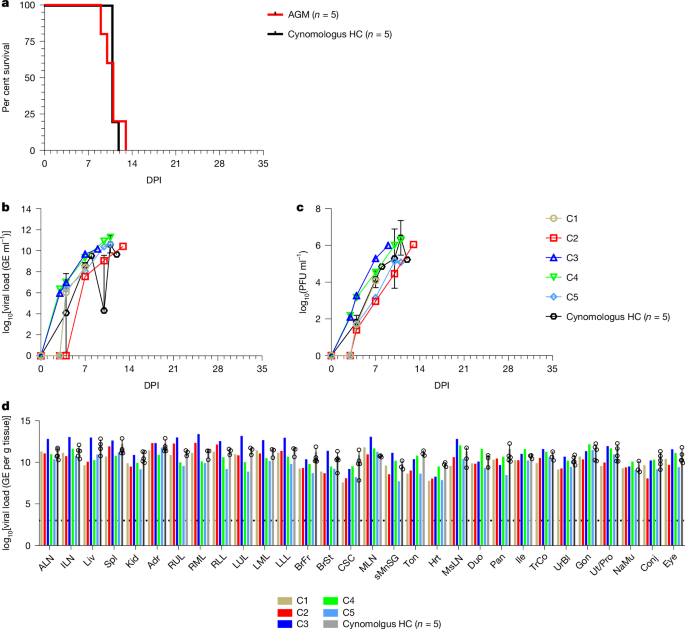
This Nature study tests the oral antiviral 4′-fluorouridine (4′-FIU, also called 4′-FlU/EIDD-2749) in an African green monkey (AGM) model challenged with a highly pathogenic lineage VII Lassa virus (LASV Togo). Five AGMs received oral 4′-FIU (5 mg kg−1 once daily for ten days) starting at day 6 post-infection, when animals were viraemic and symptomatic. All treated animals survived to the 35-day end-point, while untreated controls succumbed around days 10–12. Treated monkeys showed rapid declines in circulating infectious virus (4 of 5 cleared by day 35), developed binding and modest neutralising antibody responses, and displayed transcriptomic signatures consistent with controlled inflammation and recovery. Pathology and immunohistochemistry showed minimal lesions or viral antigen in most treated animals. The authors conclude 4′-FIU is a promising oral post-exposure therapeutic for Lassa fever that merits further development.
Key Points
- Oral 4′-fluorouridine given at 5 mg kg−1 daily for 10 days starting at day 6 post-infection rescued 5/5 treated AGMs from lethal LASV lineage VII disease; pooled untreated controls died (mean time to death ≈ 11 days).
- Treated animals rapidly reduced circulating infectious virus; 4 of 5 had no detectable infectious LASV at the 35-day end-point, one showed declining but persistent viral RNA and low infectious titres.
- Treated AGMs developed LASV glycoprotein-specific IgG and low-to-moderate neutralising titres by day 35; transcriptomics showed reduced acute inflammatory signatures and increased signals for platelets, monocytes and T-cell responses during recovery.
- Gross pathology, histology and IHC were largely absent or minimal in treated animals compared with widespread necrotising lesions and IHC positivity in controls.
- 4′-FIU is an oral ribonucleoside analogue with broad activity against several RNA viruses; here it demonstrates post-exposure efficacy in a stringent NHP model of an important viral haemorrhagic fever.
- Concerns remain about residual viral RNA or rare tissue persistence, the precise therapeutic window (treatment here began at day 6), and whether dosing/regimen can be optimised or combined with antibodies for extended protection.
Context and relevance
Lassa virus is endemic in West Africa and can cause severe, sometimes fatal, haemorrhagic fever; there are currently no licensed vaccines or reliable oral therapeutics. Previous promising treatments (favipiravir, monoclonal antibodies) require intravenous delivery or have mixed clinical evidence. An effective oral antiviral that works when given at an advanced disease stage would be a major step for outbreak response, easier deployment in low-resource settings, and could complement vaccines and antibody therapies.
Why should I read this?
Short version: this paper shows an oral pill actually saves monkeys from an otherwise lethal Lassa infection started late in illness. If you care about outbreak prep, drug development or practical treatments that don’t need IVs and cold chains, this is the one to skim — the team tested a tough virus strain in a rigorous primate model and got striking results.
Author’s take (punchy)
Big deal. Oral 4′-FIU stops bleeding-edge Lassa disease in primates when given after symptoms appear. That’s the kind of advance that could shift how outbreaks are managed — from logistically heavy IV therapies to something deployable at scale. Still early days: tissue persistence in one animal and the single dosing/timing tested mean more work is needed before human trials, but this is a clear go-ahead for accelerated development.
Caveats and next steps
Results are preclinical: NHP data are highly informative but not conclusive for humans. The study began treatment at day 6; it’s unknown how late treatment can start and still be effective. One treated animal had persistent viral RNA/infectious virus in tissues at study end, so longer follow-up and combination strategies (antiviral + antibody) should be evaluated. Dose optimisation, shorter regimens and efficacy across diverse LASV lineages and exposure routes (mucosal, aerosol) also require testing.