Stress controls heterochromatin inheritance via histone H3 ubiquitylation
Summary
This Nature study identifies a stress‑responsive regulatory hub (HRH) that controls heterochromatin propagation in Schizosaccharomyces pombe. The hub centres on the DCAF protein Raf1, a ClrC complex subunit, whose abundance is tuned by nonsense-mediated mRNA decay (NMD) and by TORC2 signalling. Raf1 promotes ClrC chromatin association and directs ubiquitylation of histone H3 at lysine 14 (H3K14ub). H3K14ub stimulates Clr4 (SUV39H) methyltransferase activity, increasing H3K9me3 density and enabling the read–write mechanism that spreads and epigenetically maintains heterochromatin. Environmental inputs (for example, caffeine, temperature and TORC2/Gad8 activity) alter Raf1 levels and thereby modulate heterochromatin robustness, with consequences for adaptive gene silencing and antifungal resistance.
Key Points
- The authors define a heterochromatin regulatory hub (HRH) centred on Raf1 DCAF that integrates environmental signals to control heterochromatin propagation.
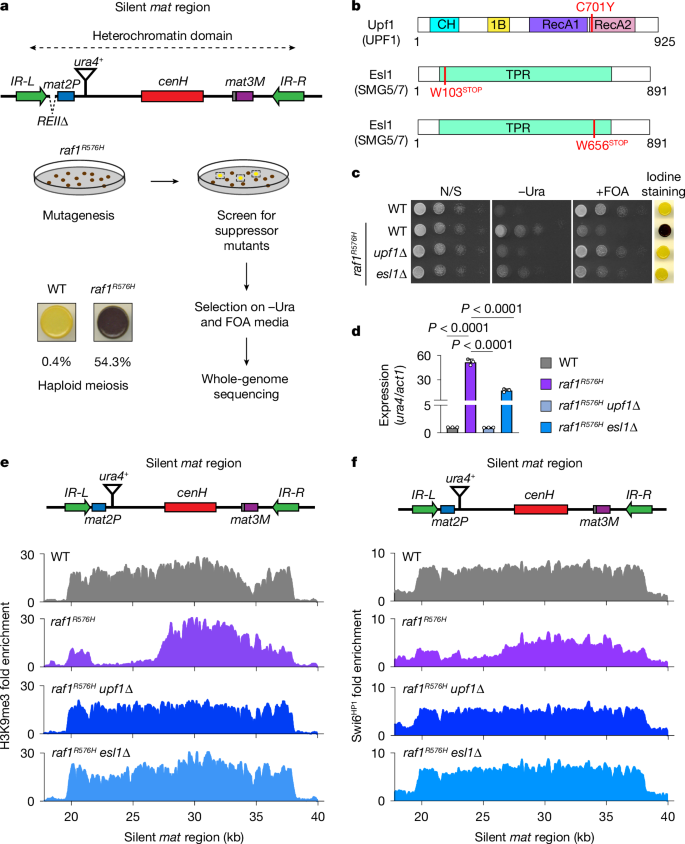
- NMD components (Upf1, Upf2 and Esl1) and the splicing factor Sap49 limit raf1 mRNA; loss of NMD or Sap49 mutations increase Raf1 and rescue spreading defects in a low‑expression raf1 R576H mutant.
- Raf1 abundance is limiting for assembly and chromatin association of the ClrC complex and for recruitment of Clr4 SUV39H methyltransferase.
- ClrC (via Raf1) monoubiquitylates H3K14 (H3K14ub), a mark required in vivo for efficient H3K9me3 spreading; Ubc4 mutation that reduces H3K14ub blocks spreading even when nucleation occurs.
- H3K14ub stimulates Clr4 enzymatic activity, raising local H3K9me3 above the critical density needed for read–write self‑propagation of heterochromatin.
- Environmental cues regulate Raf1: caffeine attenuates NMD and raises Raf1 (promoting heterochromatin and drug resistance), whereas heat inactivates TORC2/Gad8, lowering Raf1 and weakening heterochromatin inheritance.
- Increasing Raf1 (overexpression or upf1Δ) bypasses requirements for Clr3 HDAC and several chromatin factors, restores heterochromatin spreading across diverse mutants and permits sequence‑independent self‑propagation at ectopic sites.
- The pathway has broader implications: H3K14ub is conserved and the HRH may contribute to adaptive epigenetic reprogramming and antifungal resistance in other species.
Context and relevance
Heterochromatin (H3K9me3/HP1) is central to stable gene repression, cell identity and adaptive transcriptional programmes. Previous work showed histone deacetylation preserves H3K9me3 density by suppressing turnover; this paper adds a complementary mechanism: a ubiquitin ligase axis that writes H3K14ub to amplify Clr4 activity and stabilise read–write propagation. By linking NMD and TOR signalling to Raf1 levels, the study explains how growth conditions and stress rapidly tune heterochromatin robustness — a mechanism relevant to fungal drug resistance and likely conserved across eukaryotes because H3K14ub and ClrC‑like components are widely present.
Why should I read this
Short version — this paper shows stress doesn’t just change transcription: it tweaks mRNA surveillance and a ubiquitin ligase subunit (Raf1) to flip a switch on heterochromatin maintenance. If you care about epigenetic memory, stress responses, antifungal resistance or chromatin cross‑talk, the experiments and mechanisms here save you a lot of head‑scratching. There are clear, testable links from environment → Raf1 levels → H3K14ub → Clr4 activity → epigenetic inheritance.
Author style
Punchy: the authors present a convincing, multi‑angle dissection (genetics, ChIP‑seq, single‑molecule tracking, biochemistry) that elevates Raf1 as a bona fide control node for heterochromatin heritability. This is significant — it gives a mechanistic handle for how cells rapidly reinforce or relax epigenetic states in response to stress.