The astrocytic ensemble acts as a multiday trace to stabilize memory
Article Date: 15 October 2025
Article URL: https://www.nature.com/articles/s41586-025-09619-2
Article Image: Figure 1 (Nature)
Summary
This Nature study describes brain-wide, behaviour-epoch-specific tagging of astrocytes and shows that a subset of astrocytes—behaviourally relevant astrocyte ensembles (BAEs)—is specifically recruited by memory recall (not just initial learning). The authors developed a whole-brain Fos-based TRAP tagging approach (AAV-PHP.eB + TRAP2) and used serial two-photon tomography, fibre photometry, scRNA-seq and RNAscope to map, profile and perturb BAEs. They find that recall of contextual fear drives prolonged noradrenaline (NA) release and astrocyte cAMP signalling in the amygdala, and that an experience-dependent, multiday upregulation of adrenoreceptors (Adra1a, Adrb1) primes astrocytes so recall—but not initial conditioning—robustly induces Fos in an astrocyte ensemble. Those astrocytes express Igfbp2 and other effectors; manipulating astrocyte NA signalling, Adra/Adrb expression, IGFBP2 or GPCR output (iβARK2) alters reconsolidation-dependent memory stability and precision.
Key Points
- Authors created a brain-wide, astrocyte-selective Fos-tagging pipeline (AAV-PHP.eB + GfaABC1D promoter + TRAP2) enabling permanent genetic access to recall-activated astrocytes (BAEs).
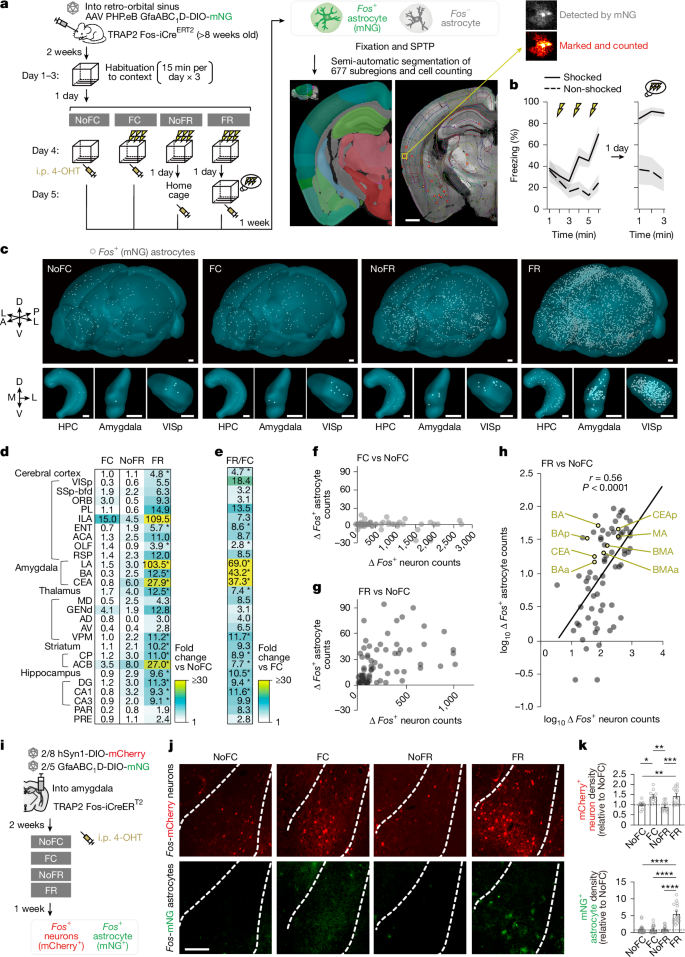
- Recall (fear recall, FR) but not habituated conditioning (FC) markedly increases Fos+ astrocyte ensembles across many regions—especially the amygdala—correlating with local neuronal engram activation.
- Mechanism: convergence of local engram activity and locus coeruleus-derived noradrenaline drives β-adrenoceptor–cAMP signalling in astrocytes; cultured and in vivo experiments support β1/α1 involvement and cAMP dependence.
- After conditioning astrocytes upregulate Adra1a and Adrb1 over days (a multiday molecular trace) that creates a window (peaking ~1 day) when recall recruits BAEs strongly.
- Astrocyte-produced IGFBP2 is enriched in Adrb1+ BAEs and contributes to memory re-stabilisation; IGFBP2 neutralisation or astrocyte GPCR inhibition (iβARK2) impairs reconsolidation-driven memory stabilisation.
- Astrocyte-specific Adrb1 overexpression amplifies BAEs and enhances memory stabilisation under weak training but can produce over-stabilisation/generalisation of fear (reduced precision).
- Functional perturbations (propranolol, LC or amygdala silencing, engram silencing, receptor cKO) reduce FR-BAE induction and disrupt memory stabilisation—showing necessity of both NA and local engram activity.
Content summary
The team first validated whole-brain astrocyte tagging, demonstrating astrocyte-selective reporter expression and a narrow temporal window (around 0–6 h with 4-OHT) for capturing behaviour-epoch Fos activity. Using contextual fear conditioning with prior habituation, they compared: NoFC, FC (conditioning + tag), NoFR (conditioned but no recall before tag) and FR (recall + tag). Serial two-photon tomography quantified Fos-tagged astrocytes across ~677 regions (~80% of brain). FR produced a much larger astrocytic ensemble than FC, particularly in amygdalar subregions, and astrocyte Fos correlated with neuronal Fos during recall.
Pharmacology and cell assays showed noradrenaline robustly induces c-Fos in astrocytes via β-receptor → adenylate cyclase → cAMP, and that co-signals (eg glutamate via mGluR3 or Ca2+ transients via α1) potentiate the response. In vivo dual stimulation of locus coeruleus (LC) and local amygdala neurons produced strong astrocyte Fos; blocking β-receptors or silencing LC or engram neurons reduced FR-BAEs. Fibre photometry revealed that recall evokes larger, prolonged NA release and progressive astrocyte cAMP increases—blocked by propranolol—matching the recall-specific Fos induction timing.
Single-cell transcriptomics and RNAscope showed Adra1a and Adrb1 upregulation following conditioning with a multiday time course (Adrb1 increased ~1.5h–3 days, Adra1a dynamics differ), producing a peak co-expression ~1 day post-conditioning that predicts FR-BAE induction. Astrocyte-specific receptor knockdown reduced FR-BAE density. Igfbp2 emerged as a downstream, secreted effector enriched in Adrb1+ astrocytes; local neutralisation of IGFBP2 during the reconsolidation window impaired memory stabilisation. Finally, selective inhibition of GPCR signalling in tagged BAEs (iβARK2) reduced Igfbp2 expression and produced weaker reconsolidation-driven freezing, while Adrb1 overexpression increased BAEs and could induce memory over-stabilisation/generalisation.
Context and relevance
This paper shifts part of the memory-reconsolidation narrative from neurons alone to astrocyte–neuron ensembles. It provides mechanistic evidence that astrocytes form a multiday molecular ‘eligibility’ trace—through adrenoreceptor priming and recall-evoked NA→cAMP signalling—that gates recall-dependent stabilisation of memory. The work links neuromodulation (LC/NA), astrocyte transcriptional state, secreted astrocyte factors (IGFBP2) and behavioural outcomes (reconsolidation vs destabilisation, precision vs generalisation).
Implications span basic memory theory (engram dynamics, spaced learning, eligibility traces) and translational avenues: targeting astrocyte–NA signalling or IGFBP2 could modulate reconsolidation in conditions such as PTSD or anxiety disorders where hyperactive noradrenergic systems and maladaptive memory stabilisation are involved.
Why should I read this?
Short and blunt — if you care about how memories stick (or why some won’t quit), this paper is gold. It shows glia aren’t just background noise: astrocytes carry a slow, multi-day molecular trace that decides whether a recalled memory gets re-stamped or weakened. Big methods, clear manipulations, and behavioural readouts mean the claims are actionable — whether you do basic engram work, neuromodulation studies, or are thinking about therapeutic reconsolidation.
Author style (punchy)
Punchy: the authors deliver a compelling, multi-technique story that amplifies astrocytes from supportive players to active regulators of memory stability. Results are highly relevant for anyone studying engrams, neuromodulation, reconsolidation mechanisms or memory-related pathology — and they point to new molecular targets (astrocytic β1/α1 receptors, IGFBP2) for modulating memory persistence and precision.