NIST Releases Reference Material to Aid Gut Microbiome Research
Summary
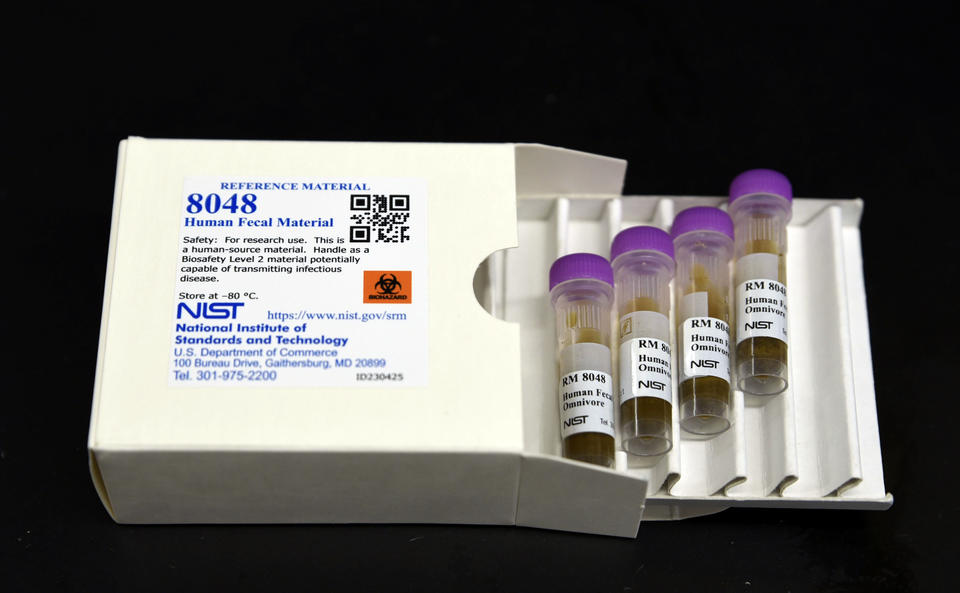
NIST has published a thoroughly characterised human stool reference material (RM 8048) intended to standardise and improve reproducibility in gut microbiome research. The kit contains eight frozen 100 mg vials — four from vegetarian donors and four from omnivores — and is accompanied by more than 25 pages of analytical data detailing identified microbes and biomolecules.
Punchy take: this is the most precisely measured human faecal standard yet and it could be the benchmark the microbiome field has been missing.
Key Points
- RM 8048 comprises eight 100 mg frozen vials (four vegetarian, four omnivore) with an expected shelf life of at least five years.
- NIST identified over 150 microbial species and more than 150 metabolites in the material using extensive genetic and chemical analyses.
- The reference material includes detailed characterisation data (25+ pages) to serve as a benchmark for labs and companies.
- Primary aims are to improve accuracy, comparability and reproducibility across different microbiome measurement methods.
- Applications include method comparison, quality control, and supporting development of live microbial therapies and faeces-derived medicines.
- The material was produced after six years of work, involving controlled donor cohorts and rigorous stability and homogeneity testing.
Why should I read this?
Short and blunt: if you care about reproducible microbiome science or are working on microbiome-based drugs, this saves you guesswork. NIST’s RM gives labs a common yardstick so results from different teams actually line up — which means faster, more reliable progress towards therapies that use live microbes or faeces-derived products.
Context and Relevance
The gut microbiome is linked to a wide range of conditions — from C. difficile infection (where faeces-derived treatments already have approved drugs) to obesity, diabetes, cancer and mental health. But inconsistent methods have hampered reproducibility and slowed translation into reliable treatments. By providing a well characterised standard, NIST aims to reduce variability between laboratories and measurement techniques, helping researchers, biotech firms and regulators compare approaches, validate assays and accelerate development of targeted microbial medicines.
The RM is especially relevant as live microbial therapies move from exploratory studies to clinical development: standard materials underpin regulatory confidence, method harmonisation and trust in biomarker measurements.