Enteropathogenic bacteria evade ROCK-driven epithelial cell extrusion
Article Date = 22 October 2025
Article URL = https://www.nature.com/articles/s41586-025-09645-0
Article Title = Enteropathogenic bacteria evade ROCK-driven epithelial cell extrusion
Article Image = https://media.springernature.com/lw685/springer-static/image/art%3A10.1038%2Fs41586-025-09645-0/MediaObjects/41586_2025_9645_Fig1_HTML.png
Summary
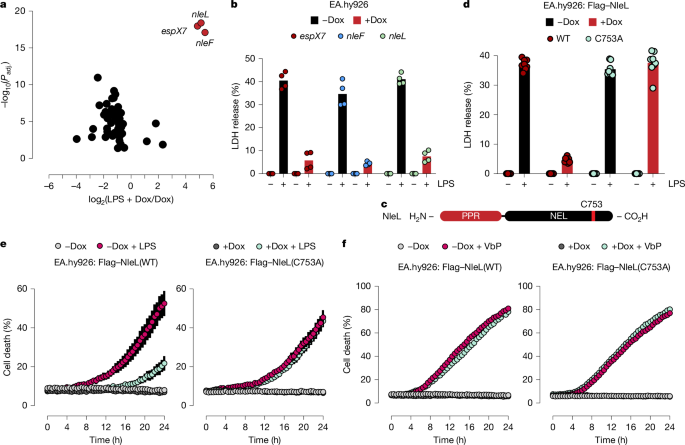
This Nature study identifies the E. coli HECT-like E3 ubiquitin ligase NleL as a virulence factor that protects enteropathogenic bacteria from host epithelial defences. NleL ubiquitylates and promotes proteasomal degradation of the LPS-sensing inflammatory caspases (caspase-4, -5 and mouse caspase-11) and the cytoskeletal kinases ROCK1 and ROCK2. By removing the caspases, it blocks non-canonical inflammasome-driven pyroptosis; by depleting ROCKs it prevents ROCK-driven extrusion of dying intestinal epithelial cells (IECs). The mechanism was shown across cell lines, primary IEC organoid monolayers and mouse infection models, and depends on NleL’s catalytic ubiquitin-ligase activity.
Key Points
- NleL is an EHEC/EPEC HECT-like E3 ubiquitin ligase that suppresses LPS-induced, caspase-4-dependent pyroptosis.
- Proteomics and biochemical assays show NleL directly ubiquitylates caspase-4 and ROCK2 (and reduces ROCK1), driving K48-linked polyubiquitylation and proteasomal degradation.
- NleL recognises LPS-sensing CARD domains in caspases (caspase-4/5/11) and the PH domain of ROCK2 as substrate determinants.
- Loss of ROCK1/2 impairs ROCK–MLC2 signalling and reduces the extrusion of pyroptotic IECs; NleL reduces extrusion and boosts bacterial colonisation in vivo.
- The ubiquitin-ligase catalytic cysteine in NleL is essential; catalytically inactive NleL(C753A) cannot induce substrate degradation or block extrusion.
Content summary
The authors screened a library of 63 E. coli type III effectors for inhibitors of intracellular LPS-triggered pyroptosis. NleL emerged as a strong hit. Mass-spectrometry-based ubiquitylome profiling identified caspase-4 and ROCK2 among the top NleL ubiquitylation targets; biochemical and in vitro ubiquitylation assays confirmed direct modification. Degradation of these proteins required the ubiquitin system and the proteasome, and was blocked by inhibitors such as MLN7243 and bortezomib. Domain mapping showed NleL targets the CARDs of LPS-sensing caspases and the PH domain of ROCK2. Functionally, NleL expression reduced ROCK signalling and limited the extrusion of infected IECs in organoid monolayers; ΔnleL bacteria showed reduced colonisation and dissemination in mice, an effect lost in mice with IEC-specific Rock1/2 deletion. The study therefore demonstrates a two-pronged bacterial strategy: prevent LPS sensing (remove caspases) and prevent mechanical expulsion (remove ROCKs).
Context and relevance
This work adds to a growing list of bacterial effectors that disarm host cell-death pathways to aid colonisation (e.g. Shigella IpaH7.8, OspC3). It reveals a previously unappreciated virulence strategy: combining blockade of inflammatory caspase sensing with suppression of the cytoskeletal machinery that physically ejects infected enterocytes. For researchers in mucosal immunology, host–pathogen interactions and drug discovery, the study highlights NleL and its enzyme–substrate interfaces as potential targets for interventions that restore epithelial defence or limit pathogen persistence.
Why should I read this?
Short answer: because it explains how E. coli gets around two of the gut’s best defences in one clever move. If you care about gut immunity, bacterial virulence or why infections stick around, this paper saves you time — it shows the molecular trick (NleL ubiquitylation) and backs it up in organoids and mice. Plus, the dual targeting (caspases + ROCKs) is neat, tidy and opens a route to target the bacterial enzyme rather than the host immune response.