Neuroendocrine control of calcium mobilization in the fruit fly
Summary
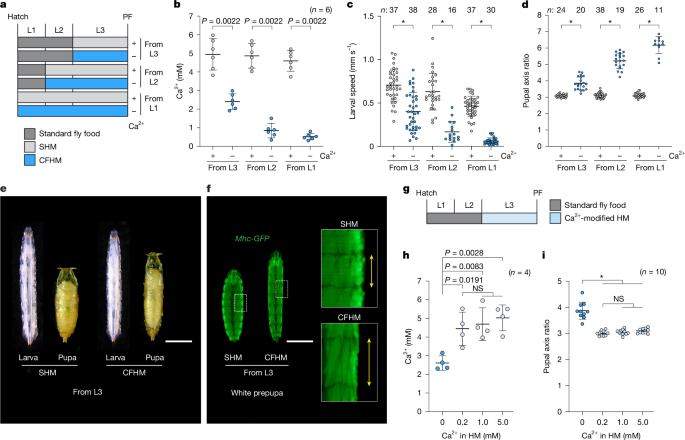
This Nature paper reports a neuroendocrine system in Drosophila larvae that maintains extracellular calcium (Ca2+) homeostasis. The authors identify Capa peptides (Capa-1 and Capa-2), produced by three pairs of ventroabdominal neurons (VaNs), as endocrine factors that act on the Capa receptor (CapaR) in the initial segments (IS) of the anterior Malpighian tubules (A-MT). The IS stores Ca2+ in pearl-like calcium granules (PCGs). Capa peptides trigger a Gαq–PKC signalling cascade in IS principal cells, mobilising Ca2+ from these granules into the haemolymph. Loss of Capa or CapaR causes hypocalcaemia, reduced locomotion, elongated pupae and increased mortality under dietary Ca2+ deficiency; conversely, dietary Ca2+ supplementation rescues these phenotypes.
Key Points
- Capa peptides from VaNs regulate haemolymph Ca2+ by acting on CapaR in the IS of the anterior Malpighian tubules.
- The IS contains pearl-like calcium granules that store roughly half the larval body’s Ca2+ and act as an internal reservoir.
- Capa-1 and Capa-2 directly mobilise stored Ca2+ from tubules ex vivo; PK-1 does not.
- Signalling downstream of CapaR requires Gαq (CG17759) and PKCε (Pkc98E) to trigger Ca2+ release.
- Knockdown of Capa or CapaR causes hypocalcaemia, neuromuscular dysfunction (reduced locomotion, impaired muscle Ca2+ transients) and elongated pupal morphology; dietary Ca2+ rescues these effects.
- The mechanism functions as an insect analogue to vertebrate PTH–PTHR systems, showing convergent endocrine solutions to terrestrial Ca2+ regulation despite different storage organs (MT vs bone).
Context and relevance
This work reveals how boneless terrestrial invertebrates maintain extracellular Ca2+ when diet is limiting. By identifying a discrete neuronal source (VaNs), a circulating peptide signal (Capa-1/2), a dedicated receptor and a storage organ (IS of the A-MT), the study fills a major gap in our understanding of insect mineral homeostasis. The findings link neuropeptide signalling to renal-like organ function, propose a conserved functional parallel with vertebrate parathyroid hormone, and provide a clear molecular pathway (Capa→CapaR→Gαq→PKC) that can be probed in other insects. The study is relevant to researchers in endocrine biology, renal physiology, insect physiology and evolutionary biology, and it opens avenues to study Ca2+ sensing mechanisms in invertebrates.
Why should I read this?
Quick answer: because it’s clever. The authors show that fruit flies don’t need bones to manage blood calcium — they use kidney-like tubules and a tiny peptide hormone circuit. If you care about how animals cope with mineral scarcity, or about neuroendocrine control of physiology, this paper gives you a neat, experimentally solid mechanism (neurons → hormone → tubule reservoir) with clear phenotypes and rescue experiments. It’s short on waffle and heavy on convincing data.
Author style
Punchy — the work is presented as a crisp discovery with immediate functional consequences (behaviour, development, survival). If you’re into mechanisms, the authors make the case that this is a broadly important, evolutionarily convergent solution to Ca2+ homeostasis; if the topic matters to you, the Results are worth reading in full for the genetic and physiological evidence.