Structural snapshots capture nucleotide release at the μ-opioid receptor
Article Meta
Article Date: 05 November 2025
Article URL: https://www.nature.com/articles/s41586-025-09677-6
Article Title: Structural snapshots capture nucleotide release at the μ-opioid receptor
Article Image: Figure 1 (cry oEM)
Summary
This Nature study uses pharmacology, single-particle cryo-electron microscopy and molecular dynamics to map the early steps of G protein activation at the μ-opioid receptor (MOR). The authors capture six structural states of MOR–Gαi complexes (inactive, latent, engaged, unlatched, primed and nucleotide-free), including four GDP-bound intermediates, and link ligand efficacy to changes in GDP affinity and release. The work explains how antagonists, partial agonists and full/superagonists differentially stabilise conformations that promote or impede GDP release — the rate-limiting step for G protein activation.
Key Points
- The team combined nuc-BRET biosensor assays, radioligand binding, cryoEM and MD simulations to study MOR–G protein interactions.
- Ligand efficacy correlates with decreased GDP affinity at Gαi: higher efficacy ligands (e.g. loperamide) markedly raise GDP EC50, while antagonists/inverse agonists (e.g. alvimopan) preserve or increase GDP affinity.
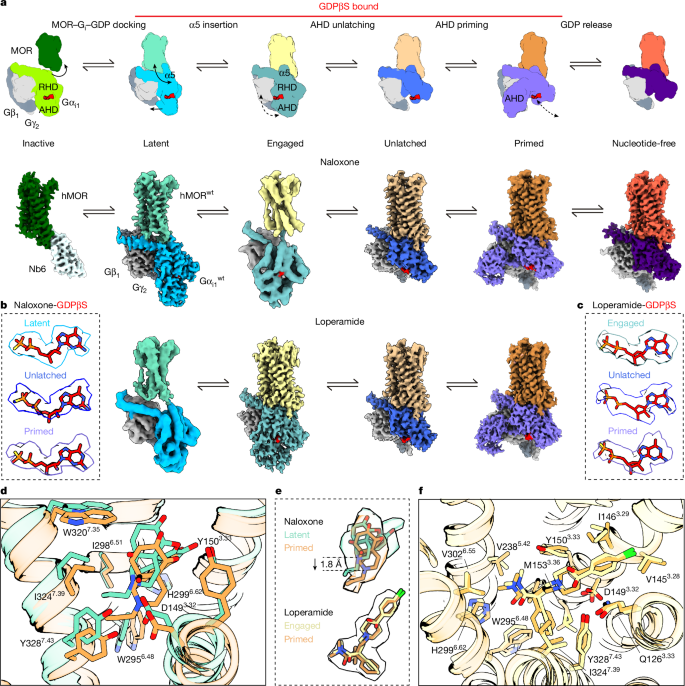
- CryoEM resolved six conformational states along the activation pathway and four distinct GDP-bound snapshots (latent, engaged, unlatched, primed) that progressively lose GDP-coordinating contacts.
- The latent state is a novel early receptor–G protein interface (G protein rotated ~60°) engaging all three intracellular loops; mutagenesis confirms its physiological relevance.
- A sequential mechanism is proposed: α5 helix insertion → AHD (α-helical domain) opening → progressive loss of GDP contacts → GDP release; MD trajectories capture transient AHD openings and occasional GDP ejection.
- Ligand binding pose (shallow for antagonists, deeper for agonists) correlates with which MOR–G protein state is stabilised and thus with GDP release rate and overall signalling efficacy.
Content Summary
The authors first clarified naloxone’s behaviour at MOR using multiple BRET-based and cAMP assays, finding it acts as a low-efficacy partial agonist (10–30%). They developed a nuc-BRET approach to measure nucleotide affinities of receptor–G protein complexes and showed a strong inverse correlation (R2 = 0.86) between signalling efficacy and GDP affinity.
Guided by the nuc-BRET data, they prepared MOR–Gαi complexes for cryoEM in conditions that allowed GDP to be observed (two purification protocols: “re-bound GDP” and “constant GDP”). CryoEM reconstructions revealed a sequence of conformational states preceding nucleotide release; the latent state is an early engagement where many GDP contacts remain, whereas engaged, unlatched and primed states progressively disrupt GDP coordination until release becomes possible.
MD simulations of each cryoEM-derived state supported the structural interpretation: the AHD is dynamically opening in the engaged and later states, and several trajectories show guanine-group displacement or full GDP ejection. Ligand poses reflected efficacy: naloxone sits more shallowly in inactive conformations but can adopt a deeper pose in active-like states; loperamide is seen in active-like poses that favour progression to GDP release.
Context and Relevance
This work addresses a central mechanistic gap in GPCR pharmacology: how ligand efficacy is transduced into G protein activation. By providing high-resolution snapshots of GDP-bound intermediates and linking them to functional nucleotide-affinity measurements, the study gives a structural basis for why partial agonists produce submaximal responses and why antagonists/inverse agonists block GDP exchange. These insights are directly relevant for rational drug design of safer opioid ligands and for broader GPCR-targeted therapeutics aiming to tune efficacy and bias.
Why should I read this?
Quick and dirty: if you care about how opioids, GPCR signalling or rational ligand design actually work at the molecular level, this paper nails the missing steps. It shows the exact conformations MOR and its G protein adopt while GDP is still bound — and how different drugs push the complex one way or another. Saves you the time of digging through scattered biochemistry and modelling papers; the cryoEM+MD combo here gives a clear road map for designing ligands that steer nucleotide exchange rates.
Author style
Punchy: the authors don’t just present structures — they stitch functional assays, mutagenesis and simulations into a coherent mechanistic model that amplifies the importance of GDP release as the central control point for ligand efficacy. If you’re interested in translating structure into safer opioid pharmacology or tuning GPCR signalling more generally, read the details.