Anti-progestin therapy targets hallmarks of breast cancer risk
Article meta
Article Date: 05 November 2025
Article URL: https://www.nature.com/articles/s41586-025-09684-7
Article Image: Figure 1
Summary
This study (BC-APPS1, NCT02408770) tested 12 weeks of ulipristal acetate (UA; 5 mg/day) in premenopausal women at elevated lifetime breast‑cancer risk. Paired biopsies, multi-OMICs, imaging and functional assays show that short-term anti-progestin treatment:
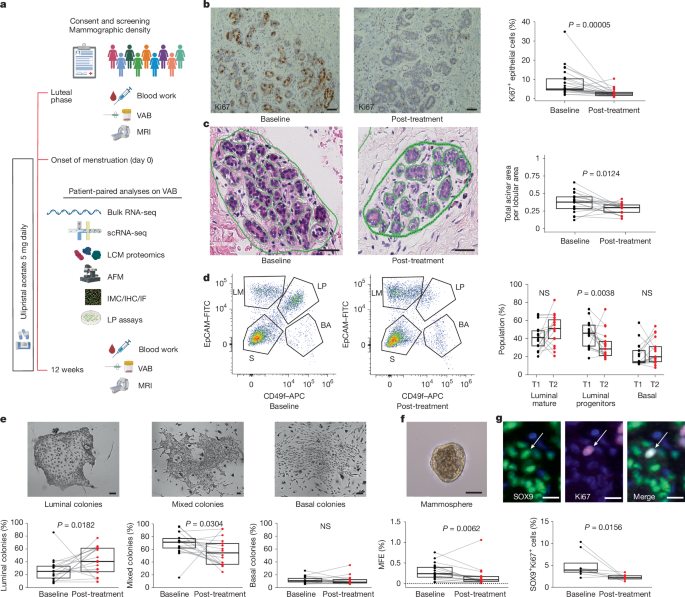
– markedly lowers epithelial proliferation (Ki67 fell from a median ~8.2% to ~2.9%),
– reduces the proportion, proliferation and functional activity of luminal progenitor/LASP (SOX9+) cells (the putative precursor pool for some aggressive tumours),
– remodels the extracellular matrix: reductions in multiple collagen genes and matrisome proteins (notably COL6A2/COL6A3) and decreased collagen fibre alignment,
– reduces tissue stiffness by AFM and lowers fibroglandular volume (FGV) by MRI — a radiological surrogate for mammographic density,
– shows concordant mechanistic data in organoid/stiffness assays where anti-progestins block stiffness-amplified progesterone signalling (SOX9, KIT) and progenitor activity.
Treatment was generally well tolerated in this short study (24 paired molecular analyses), but the authors note the need for longer-term safety data (especially liver and endometrial effects) and definitive trials to show risk reduction.
Key Points
- BC-APPS1 was a single-centre phase II window trial in premenopausal women at increased breast-cancer risk; 24 participants had paired analyses.
- 12 weeks of ulipristal acetate significantly reduced Ki67 proliferation in normal breast epithelium (median ~8.2% to ~2.9%, P < 0.0001).
- Anti-progestin therapy decreased luminal progenitor/LASP cell frequency, clonogenic/mammosphere activity and SOX9+ proliferative cells — the likely origin of some triple‑negative cancers.
- Multi-OMICs (scRNA-seq, bulk RNA-seq, proteomics) and CellChat/NicheNet analyses point to reduced LHS (luminal hormone-sensing) cell ligands and downstream downregulation of fibroblast/basal matrisome genes, especially collagens.
- Spatial proteomics and AFM confirm ECM remodelling: lower collagen I/VI and fibronectin near SOX9high cells, decreased collagen alignment and reduced peri-lobular stiffness.
- MRI-measured fibroglandular volume (FGV), a surrogate for mammographic density, was reduced after 3 months of UA; women with higher baseline density showed greater epithelial proliferative reductions.
- In vitro, increasing matrix stiffness amplifies progesterone signalling and progenitor activity; anti-progestins block that stiffness-driven effect, showing a plausible mechanistic feedback loop between ECM stiffness and PR signalling.
- Short-term UA appears effective at modulating biologically plausible surrogates of breast-cancer risk, but longer studies are required to confirm safety and actual impact on cancer incidence.
Context and relevance
Progesterone drives cyclical expansion of luminal progenitors via paracrine signalling from PR+ luminal mature cells; that progenitor pool is implicated in the origin of basal/triple-negative cancers. Epidemiological and experimental data link progestins and combined hormone therapy to increased breast-cancer incidence and higher mammographic density. This trial provides human in vivo evidence that blocking progesterone signalling alters both epithelial precursor cells and the stromal niche — reducing radiological density and the biophysical properties that sustain progenitor expansion. The work ties molecular, spatial and biomechanical data together and highlights mammographic density/FGV as potential early biomarkers of response.
Author style
Punchy: This is a strong, mechanistically rich human study that connects a druggable hormone axis to measurable changes in both the epithelial precursor pool and the tissue microenvironment. If you care about primary prevention or precision prevention strategies for premenopausal, high‑risk women, these findings matter — they show a plausible, targetable route to lower two recognised hallmarks of breast-cancer risk (progenitor activity and mammographic density).
Why should I read this?
Short answer: because it’s rare to see a human prevention study that combines clinical imaging, biopsies and multi‑OMICs and then links those findings to tissue mechanics and organoid experiments. If you’re interested in breast‑cancer prevention, how mammographic density might be drug‑modifiable, or the role of progesterone signalling in risk, this paper saves you time — it pulls the biology, imaging and early clinical proof into one place.
Limitations & next steps
Small sample, single arm, short duration. UA was suspended historically for hepatotoxicity concerns, so longer and larger safety trials are essential. Crucially, whether these surrogate changes translate into fewer cancers remains to be tested in prospective prevention trials. Biomarker-driven selection (for example, high density or PR‑signature) could enrich future studies.