Synthesis of enantioenriched atropisomers by biocatalytic deracemization
Summary
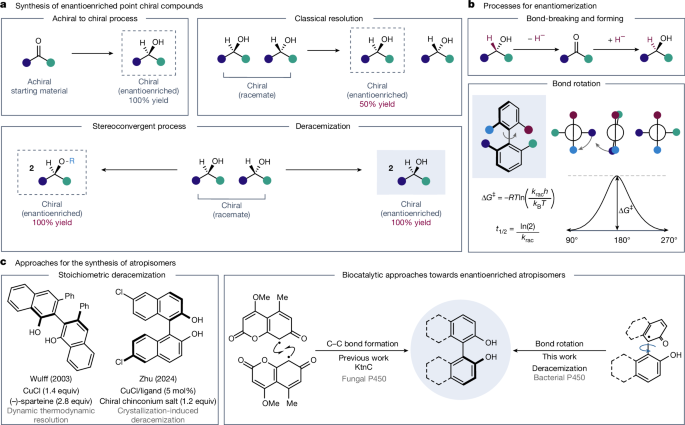
This paper reports a newly discovered enzymatic deracemization that converts racemic BINOL-type atropisomers into enantioenriched products using a cytochrome P450 (CYP158A2) scaffold engineered by directed evolution (BinDR variants). The reaction requires surprisingly simple conditions: the engineered P450, atmospheric oxygen and sodium ascorbate. Mechanistic and control experiments support a cycle in which enzymatic oxidation produces a stereolabile phenoxy radical with a much lower rotational barrier; subsequent reduction (or H-atom transfer/PCET) re-closes the chiral axis in the preferred configuration. The approach gives high enantiomeric ratios and high recovery for many substituted BINOL derivatives, and protein engineering can tune selectivity to challenging substrates.
Key Points
- A P450 (CYP158A2) and engineered variants (BinDR1–BinDR6) catalyse deracemization of BINOL atropisomers to give high enantiomeric ratios with good recovery.
- Minimal reaction components are P450 enzyme, atmospheric oxygen and sodium ascorbate; catalase abolishes activity, implicating peroxide/oxygen chemistry localised by the enzyme.
- Mechanism: enzyme-mediated oxidation forms a phenoxy radical (lowering the rotation barrier), rotation interconverts atropisomers, and reduction/PCET restores the closed-shell BINOL in an enzyme-selected configuration.
- Deracemization is net redox-neutral in the vessel (O2 as terminal oxidant, NaAsc as reductant) and benefits from excess ascorbate to limit overoxidation and enzyme inactivation.
- Protein engineering markedly improves latent deracemization activity of the wild-type enzyme and allows creation of specialist BinDR variants optimised for particular substitution patterns.
- Scope: many 3-substituted, 3,3′-disubstituted and C2-symmetric BINOLs show good enrichment, but selectivity depends strongly on substitution pattern and enzyme variant.
- Deracemization is not universal among P450s; some oxidative coupling P450s show no deracemization, indicating this is a distinct and tuneable enzymatic function.
Content summary
Atropisomers are increasingly important in drug discovery because locking a biaryl axis can improve potency and selectivity. Traditional enantioselective bond-forming methods sometimes fail or force chromatographic resolution (yield penalty). The authors discovered during an engineering campaign that a bacterial P450 (CYP158A2) and evolved variants can deracemize BINOL-type atropisomers — converting racemates into a single enantiomer by promoting bond rotation rather than bond breaking/forming.
Key experimental findings: time-course studies showed enantiomeric enrichment increasing during reaction with high recovery, inconsistent with simple kinetic resolution and supporting deracemization. Systematic controls established that the enzyme, oxygen (or peroxide) and sodium ascorbate are the minimal required players. High peroxide alone causes enzyme deactivation and substrate loss; the combination of atmospheric O2 and NaAsc gives the best recovery and controllable rates.
Mechanistically, enzyme-mediated oxidation gives a phenoxy radical intermediate whose rotational barrier is much lower (computationally estimated ~25.5 kcal mol−1 for a neutral radical), enabling interconversion at ambient temperature. The chiral active site biases the oxidation step (stereoselective step) so when the radical is reduced back to BINOL the favoured atropisomer accumulates. Directed evolution improved enantioselectivity and enabled variant-specific selectivity trends across a broad substrate panel.
Context and relevance
This work provides a complementary stereoconvergent route to enantioenriched atropisomers that avoids the 50% theoretical yield limit of simple resolution and does not rely solely on asymmetric bond construction. For medicinal chemists and ligand designers who use BINOL and related scaffolds, the method is attractive because it can deliver high enantiomeric ratios with high material recovery and is tunable by protein engineering. More broadly, it demonstrates that enzymes can control stereodynamic processes (conformational interconversion) and that latent enzymatic functions can be amplified to achieve synthetically useful transformations.
Why should I read this
Quick version: if you care about getting single-handed biaryl scaffolds without losing half your material or wrestling endless chromatography, this is tidy, clever and potentially game-changing. The team found an enzyme trick that briefly turns a rigid biaryl into a floppy radical so it can flip, then locks it back in the favoured form — and you don’t need exotic reagents, just the P450, oxygen and ascorbate. If you design chiral ligands, work on drug leads with axial chirality or tinker with biocatalysis, give this one a read — it’s a proper shortcut and shows protein engineering can tune the outcome.
Author style
Punchy: the paper reports a genuinely novel catalytic deracemization mediated by an engineered P450, with clear mechanistic evidence and practical substrate scope. For chemists working in asymmetric synthesis or biocatalysis this is highly relevant — the authors amplify that this latent enzymatic capability can be evolved into specialist catalysts to access atropisomerically enriched materials that were previously difficult or inefficient to obtain.
Source
Article Date: 12 November 2025