Tumour-reactive heterotypic CD8 T cell clusters from clinical samples
Summary
This Nature paper reports that heterotypic clusters — physical conjugates of CD8+ T cells with tumour cells and/or antigen-presenting cells (APCs) — can be isolated directly from patient melanoma specimens. Using imaging flow cytometry, FACS, combined single-cell RNA/TCR sequencing and functional assays, the authors show these clustered CD8+ T cells are enriched for tumour-reactive, clonally expanded, exhausted/proliferative states and have markedly higher anti-tumour activity ex vivo and in vivo compared with single T cells. Clusters contain specific tumour and myeloid subpopulations expressing ligands that promote immune synapse formation and T cell attraction. A transcriptional signature derived from cluster T cells predicts response to TIL therapy in an external cohort, and cluster-derived T cells outperform traditional CD39-based enrichment strategies in killing assays.
Key Points
- Heterotypic clusters of CD8+ T cells with tumour cells and APCs are detectable and isolatable from fresh clinical melanoma samples (including lymph nodes).
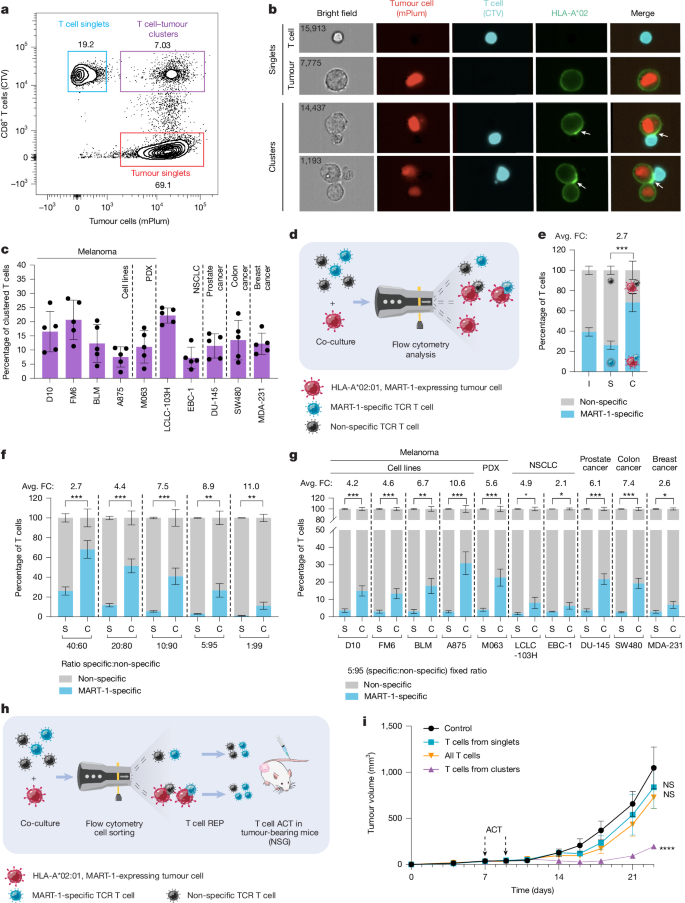
- In controlled co-cultures antigen-specific CD8+ T cells outcompete bystanders to form clusters and show immunological synapses with tumour cells.
- Single-cell RNA/TCR profiling reveals cluster-derived CD8+ T cells are more exhausted/proliferative, clonally expanded and enriched for tumour-reactive gene programmes versus singlets.
- Specific tumour states (high antigen presentation/IFN/stress) and certain APC subtypes (C1q-high macrophages, plasmacytoid and mregDCs) are preferentially engaged in clusters and express ligands that mediate attraction and synapse formation.
- T cells recovered from clusters show substantially higher cytokine production and tumour-killing ex vivo and better tumour control after adoptive transfer in PDX/NSG models.
- Cluster-derived signatures (including a TCF7+ stem-like exhausted subset) predict clinical TIL therapy response and include CD39− tumour-reactive cells often missed by CD39-based sorting.
Content summary
The authors combined short enzymatic digestion of melanoma specimens with careful low-event-rate FACS and imaging flow cytometry to identify and sort heterotypic clusters (T cell:tumour and T cell:APC). In vitro competition assays using MART-1 TCR-transduced T cells showed antigen-specific CD8+ T cells preferentially formed conjugates and relocalised synapse markers (ICAM1, CD58, HLA) to the interface.
scRNA-seq and scTCR-seq of sorted singlets and clusters from five patients revealed 14 CD8+ T cell states. Cluster-derived T cells were enriched for exhausted/proliferative and clonally expanded states with tumour-reactive transcriptional programmes; bystander/virus-reactive signatures were enriched in singlets. Tumour and APC cell states found in clusters expressed chemokines and adhesion molecules (e.g. CXCL9/10, CCL5, ICAM1, CD58) and immune-modulatory ligands (PD-L1, CD80/86).
Functionally, cluster-derived T cells expanded in REP produced more IFNγ/TNF and delivered 8–9-fold higher killing of autologous tumour cells ex vivo versus singlets. In two independent mouse models (hIL-2-NOG and NSG PDX), cluster-derived T cells infiltrated better and delayed tumour growth, while singlet-derived T cells were largely ineffective. Cluster-derived products also outperformed CD8+CD39+ enrichment in matched comparisons, and clusters contained a therapeutically favourable TCF7+ stem-like exhausted population, including many CD39− clonotypes.
Context and relevance
Spatial proximity of T cells to tumour cells has been linked to immunotherapy outcomes; this study moves from correlation to a practical approach for capturing the actual interacting (and tumour-reactive) T cell population. Because typical single-cell workflows and single-cell gating often discard multicellular conjugates, important tumour-reactive T cells may be missed. The work therefore has implications for improving TIL manufacturing, selecting TCRs for therapy, and for tissue- and single-cell-based studies that aim to map functional tumour–immune interactions.
Why should I read this?
Want the T cells that actually fight the tumour rather than noisy bystanders? This paper shows a clever, practical way to capture them straight from patient tissue. If you care about better TIL products, predictive biomarkers for therapy or finding tumour-specific TCRs, it’s a shortcut worth knowing — and it might change how people process clinical samples for single-cell work.