Fasting boosts breast cancer therapy efficacy via glucocorticoid activation
Summary
This Nature study shows that short, periodic fasting (or fasting-mimicking diets) enhances the anti-tumour effect of tamoxifen in hormone receptor-positive (HR+) breast cancer by increasing circulating corticosteroids and progesterone. Those hormonal changes activate the glucocorticoid receptor (GR) and progesterone receptor (PR) inside tumours, triggering epigenomic remodelling (notably H3K27ac changes) that represses AP-1-driven enhancers and boosts GR/PR-bound enhancers. The result is reduced proliferation programmes (MYC, E2F), elevated GR transcriptional activity (including induction of the suppressor ZBTB16), and delayed resistance to endocrine therapy in multiple preclinical models. GR knockout abolishes the fasting benefit, while short-course dexamethasone (a GR agonist) phenocopies fasting and potentiates tamoxifen in xenografts, patient-derived models and an immunocompetent allograft, though chronic corticosteroid use carries known risks. Human trial samples confirm that a five-day fasting-mimicking diet raises cortisol and progesterone and increases GR/PR activity in tumours.
Key Points
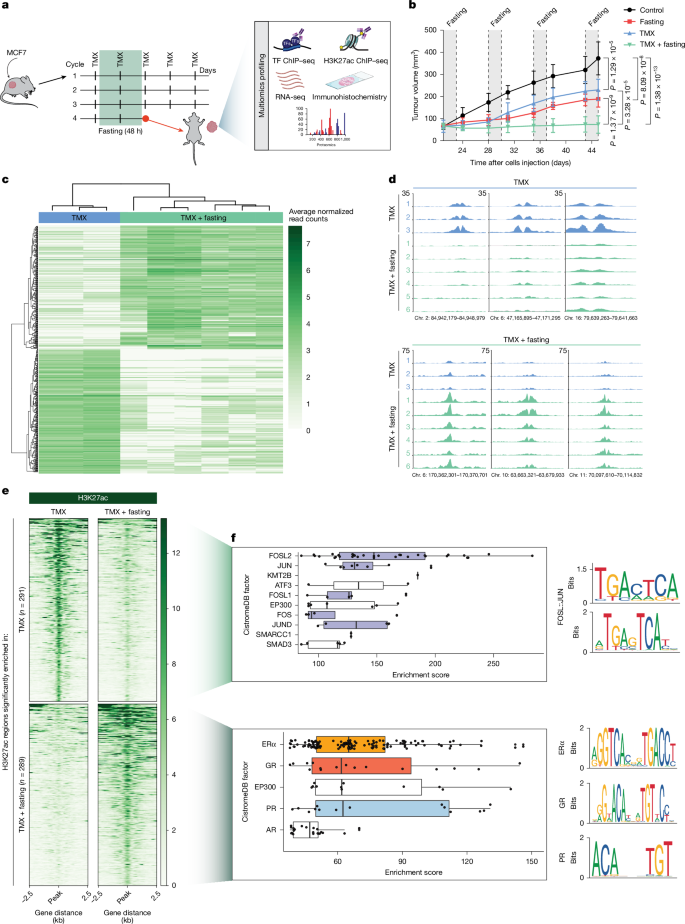
- Weekly 48 h fasting cycles synergise with tamoxifen in HR+ breast cancer xenografts, producing stronger tumour control than either alone.
- Multi-omic profiling shows fasting remodels tumour enhancers (H3K27ac) — AP-1-associated enhancers lose activity while GR/PR-occupied enhancers are gained.
- Fasting and FMD raise circulating corticosterone/cortisol and progesterone, driving intratumoural activation and nuclear localisation of GR (and increased PR binding).
- Activated GR transcriptional programmes suppress cell-proliferation signatures (MYC, E2F, G2M) and upregulate suppressors such as ZBTB16.
- Genetic loss of GR removes fasting’s benefit; conversely, dexamethasone (Dexa) mimics fasting’s effects and enhances tamoxifen efficacy across models, with a carry-over anti-tumour effect after treatment stops.
- Short-course Dexa plus tamoxifen did not produce obvious immune suppression in the tested immunocompetent model and reduced PD-L1 on several myeloid populations, but chronic glucocorticoid risks remain and clinical caution is needed.
Content summary
The authors combined in vivo xenograft experiments with deep multi-omic analyses (ChIP–seq for H3K27ac and multiple TFs, RNA-seq, proteomics) to define how fasting enhances tamoxifen response. Fasting produced marked epigenetic reprogramming: many enhancers lost H3K27ac (AP-1 motifs enriched among those) while others gained it with strong in silico and experimental evidence of ERα, GR and PR occupancy. Hormone assays in mice and in patients undergoing a five-day fasting-mimicking diet showed elevated corticosteroid and progesterone levels, correlating with increased GR/PR chromatin binding and transcriptional signatures.
Functionally, fasting plus tamoxifen downregulated proliferation pathways and mTOR signalling. GR activity rose markedly with fasting; knocking out GR in MCF7 cells abrogated the fasting benefit. Importantly, giving dexamethasone in place of fasting reproduced the synergy with tamoxifen across MCF7 and T47D xenografts, a patient-derived xenograft and in immunocompetent allografts, and delayed tumour regrowth after treatment cessation. Dexa lowered IGF1 (one fasting-reduced factor) but did not cause the weight loss seen with fasting.
The study emphasises that GR activation is a key mediator of fasting’s benefit in HR+ breast cancer and proposes short-term GR agonism as a potential fasting-mimetic strategy to enhance endocrine therapy — while noting the known adverse effects of chronic corticosteroids and the need for careful clinical evaluation.
Context and relevance
Why this matters: endocrine therapy remains central to treating HR+ breast cancer, but resistance is common. Dietary interventions such as fasting or FMDs have shown promise but are hard to sustain long term. This work identifies a clear molecular mechanism — hormone-driven GR/PR activation and epigenetic switching — that links metabolic state to therapy response, and it demonstrates a clinically available drug (dexamethasone) can reproduce the desirable anti-tumour effects of fasting in preclinical models. That opens a conceivable path to short, pharmacological fasting-mimetics to potentiate endocrine therapy and delay resistance.
Who should read it: oncologists, translational researchers and clinical trialists interested in therapy sensitisation, hormone receptor biology and metabolic interventions in cancer. It is particularly relevant to teams developing combination regimens for HR+ breast cancer and those designing trials of fasting-mimicking diets or steroid-modulating agents.
Why should I read this?
Short version — this paper explains why a simple physiological change (fasting) helps tamoxifen work better and shows a ready-made drug (dexamethasone) can copy that effect in models. If you care about making endocrine therapy last longer or finding practical ways to mimic fasting without asking patients to starve, this is the one to skim — then read the methods and figures if you want to use it clinically or translate it to trials.