GTP release-selective agonists prolong opioid analgesic efficacy
Summary
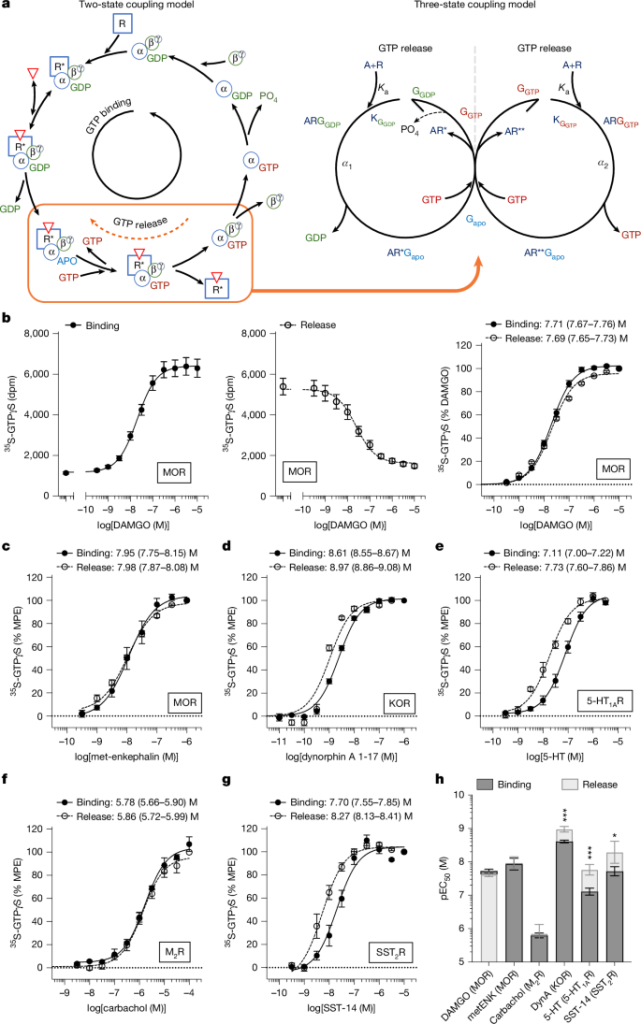
This Nature study shows that G-protein-coupled receptors (GPCRs) can drive not only GDP→GTP exchange on Gα proteins but also catalyse the release of bound GTP — a reversible, agonist-dependent exchange activity. The authors describe a three-state coupling model and demonstrate that some mu-opioid receptor (MOR) agonists preferentially promote GTP release rather than GTP binding. Two newly characterised compounds, muzepan1 and muzepan2, are highly selective for the GTP-release state. In mouse experiments these ‘release‑selective’ agonists penetrate the brain and, at low (sub‑efficacious) doses, markedly enhance and prolong morphine and fentanyl analgesia beyond additive effects, while not increasing opioid-induced respiratory suppression or bradycardia at those low doses.
Punchy take: this paper identifies a previously under-appreciated, state-selective facet of GPCR–G protein coupling and shows it can be exploited pharmacologically to boost opioid analgesia without proportionally worsening breathing or heart-rate effects — at least in the mouse models tested.
Key Points
- GPCRs can catalyse both GTP binding and GTP release from Gα subunits; the two processes are agonist-dependent and can have different potencies and efficacies.
- The authors present a three-state model capturing distinct receptor–G protein active states that explain differential agonist effects on binding vs release.
- Muzepan1 and muzepan2 are MOR agonists with strong selectivity for promoting GTP release over GTP binding (≈100-fold selectivity for release in some assays).
- In mouse spinal cord and behavioural assays, muzepans enhance and prolong morphine and fentanyl antinociception; a low dose of muzepan1 roughly doubles morphine potency (ED50 shift) and extends fentanyl efficacy over time.
- At low, potentiating doses muzepan1 does not increase fentanyl-induced respiratory depression or bradycardia; however, high doses of muzepan1 alone can suppress respiration and heart rate.
- The findings suggest a mechanism where a receptor-driven GTP release cycle keeps G proteins nearby and able to re-engage rapidly, altering downstream signalling and possibly biasing pathway engagement.
- Caveats: muzepans are probe compounds (patent applications noted), safety, addiction liability, tolerance and long-term effects are not established; translational relevance to humans requires further study.
Content summary
The authors used parallel 35S‑GTPγS binding (GDP→GTP capture) and a pulse‑chase 35S‑GTPγS release assay to measure two opposing exchange functions across several GPCRs, including MOR. They found that agonists can show different rank‑order potencies and efficacies for promoting binding versus promoting release. Several known G‑protein‑biased ligands displayed release preference, but the correlation is not absolute.
Two new MOR compounds, muzepan1 and muzepan2, were synthesised and shown in CHO cell membranes and mouse spinal cord preparations to be far more potent at inducing GTP release than binding. Both compounds are brain‑penetrant. In behavioural pain assays, sub‑analgesic doses of muzepan1 enhanced and prolonged morphine and fentanyl analgesia in mice beyond additive prediction. Pharmacokinetic and CYP inhibition tests indicate muzepans do not enhance opioid effects by impairing opioid metabolism. Importantly, at the low enhancing dose muzepan1 did not worsen fentanyl‑induced respiratory depression or bradycardia in mice, though higher muzepan doses alone produced cardiorespiratory effects.
The discussion proposes that bidirectional exchange (binding and release) changes the local G‑protein availability and kinetics, potentially explaining some forms of signalling bias and offering a new lever to modulate therapeutic windows of GPCR drugs. The authors caution that muzepans are probes and further optimisation is needed to combine release preference with other desirable properties (partial agonism, slow onset, etc.) to improve safety.
Context and relevance
Why this matters: opioid analgesics provide powerful pain relief but are limited by side effects such as respiratory depression. This paper identifies a novel, druggable dimension of MOR signalling — preferential promotion of GTP release — that can potentiate analgesia at low co‑administered doses without proportionally enhancing respiratory harm in mice. For researchers and drug developers working on analgesics, GPCR signalling bias and mechanism‑based safety, these results open a fresh strategy to amplify benefit while potentially narrowing side‑effect risk. The findings may also apply beyond opioid receptors to other GPCR drug programmes where altering G‑protein cycling could change downstream pathway engagement.
Why should I read this?
Because it’s clever and potentially useful: the team has found a new, state‑selective way to tweak GPCR signalling that actually improves opioid pain relief in animals without clearly worsening breathing at the low doses that do the boosting. If you work on pain, GPCR pharmacology or drug discovery this could save you months of guesswork — read the paper for the assays, the three‑state model and the in vivo data.